Apr 26 2024Reviewed by Lexie Corner
In a recent study published in the journal Dalton Transactions, scientists at the Shibaura Institute of Technology have made significant progress in the creation of displacement-type ferroelectric material with a high dielectric constant.
 The researchers investigated crystal structure and properties of perovskite-RbNbO3 prepared at 4 GPa. Single-crystal XRD analysis revealed RbNbO3 exhibits the same non-centrosymmetric space group as ferroelectrics BaTiO3 and KNbO3. Image Credit: Ayako Yamamoto from SIT, Japan
The researchers investigated crystal structure and properties of perovskite-RbNbO3 prepared at 4 GPa. Single-crystal XRD analysis revealed RbNbO3 exhibits the same non-centrosymmetric space group as ferroelectrics BaTiO3 and KNbO3. Image Credit: Ayako Yamamoto from SIT, Japan
Among the accomplishments is the successful synthesis of rubidium niobate (RbNbO3), a substance that was previously thought to be difficult to make at pressures higher than 40,000 atmospheres. They also described how polarization varies during phase transitions over a broad temperature range. This discovery may result in revised specifications for ferroelectric materials.
Capacitors are essential parts of electronic devices, including computers and smartphones. The dielectric materials used in construction polarize when a voltage is applied. At the moment, the most popular material for capacitors is barium titanate (BaTiO₃). Barium titanate is a material that is part of the perovskite group, in which an oxygen octahedral cage contains a titanium ion.
The substance demonstrates displacive-type ferroelectric behavior, in which a permanent dipole moment is created inside the material due to ion displacement during the phase transition.
Researchers under the direction of Professor Ayako Yamamoto from the Shibaura Institute of Technology, along with master student Kimitoshi Murase, created a displacement-type ferroelectric material with a high dielectric constant. Dr. Hiroki Moriwake and the team at the Japan Fine Ceramics Center looked into the theoretical aspect.
Researchers synthesized rubidium niobate (RbNbO3) by successfully incorporating large rubidium ions into perovskite-type compounds using a high-pressure method. This compound was successfully synthesized using an inventive method despite its difficult synthesis process in the past.
Since the 1970s, there has been interest in synthesizing RbNbO3, as it displays displacement ferroelectricity similar to BaTiO3, making it a promising candidate for capacitor designs. However, studies on its dielectric characteristics have only been conducted at low temperatures (below 27 °C). The crystal structure and phase transitions over a wide temperature range (–268 to +800 °C) are clarified by this study, opening the door for additional investigation and advancement.
The high-pressure synthesis method has reported a variety of materials with perovskite-type structures, including superconductors and magnets. In this study, our focus was on combining niobates and alkali metals known for their high dielectric properties.
Ayako Yamamoto, Professor, Shibaura Institute of Technology
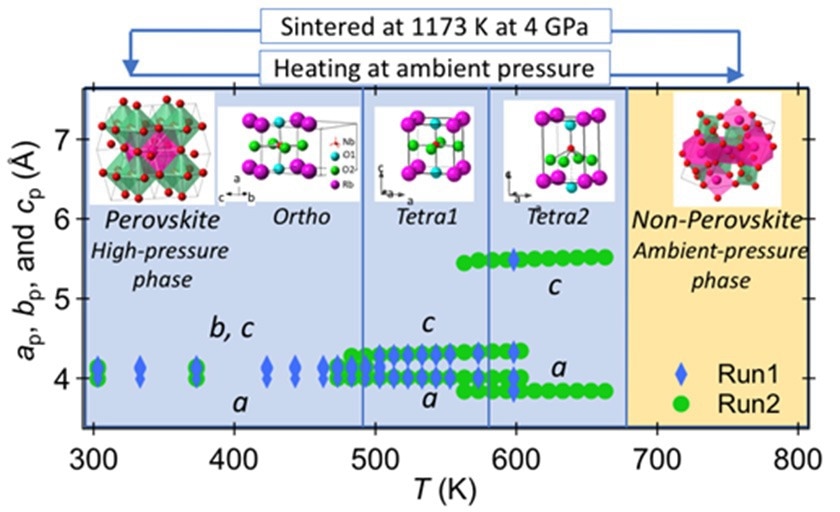
By sintering a mixture of rubidium carbonate and niobium oxide at 1073 K (800°C) and subjecting it to high pressures of 40,000 atmospheres at 1173 K (900°C) for 30 minutes, the researchers were able to create non-perovskite-type RbNbO3. The rubidium niobate underwent a structural transformation under these high-pressure and high-temperature conditions, changing from a complex triclinic phase at ambient pressure phase into a 26 % denser orthorhombic perovskite-type structure.
The researchers studied the crystal structure using X-Ray diffraction. Based on a single crystal analysis, they found that the crystal structure displayed distortions similar to those seen in BaTiO3, another well-known ferroelectric material, and was remarkably similar to that of potassium niobate (KNbO3). They discovered that in RbNbO3, niobium atom displacement and orthorhombicity were greater than in KNbO3, suggesting a greater level of dielectric polarization due to phase transitions.
Using powder X-Ray diffraction, four different phase transitions were found to occur at temperatures ranging from –268 °C to +800 °C. The most stable phase of RbNbO3 is the orthorhombic phase, which exists below room temperature. It changes phases as the temperature rises: above 220 °C, it transitions to a tetragonal perovskite phase, and above 300 °C, it becomes more elongated. Ultimately, it returns to a non-perovskite phase present in atmospheric conditions above 420 °C.
The observed phase transitions agree with the predictions of first-principles calculations. The researchers also computed the dielectric polarization of various RbNbO3 phases. The two tetragonal phases displayed polarizations of 0.4 and 0.6 Cm−2, respectively, while the orthorhombic phase displayed a polarization of 0.33 Cm−2. Ferroelectric alkali metal niobates like KNbO3 (0.32 Cm−2), LiNbO3 (0.71 Cm−2), and LiTaO3 (0.50 Cm−2) have values similar to these.
The high-pressure phase obtained this time confirmed the presence of a polar structure from the observation of second harmonic generation of the same strength as potassium niobate, and a relatively high relative permittivity was also obtained. As for the dielectric constant, it is expected that values equal to or greater than those of potassium niobate can be obtained by increasing the sample density, as predicted from theoretical calculations.
Ayako Yamamoto, Professor, Shibaura Institute of Technology
The researchers are planning more tests to precisely determine the dielectric constant and high polarization of RbNbO3. The high-pressure method’s benefit is its capacity to stabilize materials that are nonexistent at atmospheric pressure. Larger alkali metal ions, like cesium, could be added to the perovskite structure using the suggested technique, producing ferroelectrics with advantageous dielectric characteristics.
Journal Reference:
Yamamoto, A., et al. (2024) Crystal structure and properties of perovskite-type rubidium niobate, a high-pressure phase of RbNbO3. Dalton Transactions. doi.org/10.1039/D4DT00190G