|
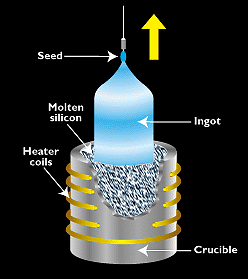
Figure 2. - The most widely used technique for making single-crystal silicon is the Czochralski process, in which seed of single-crystal silicon contacts the top of molten silicon. As the seed is slowly raised, atoms of the molten silicon solidify in the pattern of the seed and extend the single-crystal structure
The Float Zone Process
The float zone (FZ) process produces purer crystals, because they are not contaminated by the crucible as Czochralski crystals are. In the FZ process, a silicon rod is set atop a seed crystal and lowered through an electromagnetic coil. The coil's magnetic field induces an electric field in the rod, heating and melting the interface between the rod and the seed. Single-crystal silicon forms at the interface, growing upward as the coils are slowly raised.
Once the single-crystal ingots are produced, the must be sliced to form wafers.
Although single-crystal silicon technology is well developed, the Czochralski, FZ, and ingot-casting processes are complex and expensive. A group of new crystal-producing processes, however, generally called shaped-ribbon growth, could reduce processing costs by forming silicon directly into thin, usable wafers of single-crystal silicon. These methods involve forming thin crystalline sheets directly, thereby avoiding the slicing step required of cylindrical ingots.
Semicrystalline Material
Semicrystalline silicon can be produced in a variety of ways. The most popular commercial methods involve a casting process in which molten silicon is directly cast into a mould and allowed to solidify into an ingot. Generally, the mould is square, producing an ingot that can be cut and sliced into square cells to fit more compactly into a PV module. (Round cells leave space between them, where square cells can fit together with a minimum of wasted space).
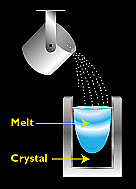
Figure 3. - The most popular method for making commercial semicrystalline silicon is casting, in which molten silicon is poured directly into a mould and allowed to solidify into an ingot.
Source: U.S. Department of Energy Photovoltaics Program.
For more information on this source please visit National Renewable Energy Laboratory
|