Mass spectrometry is a gas analysis technique that is extensively used in several respiratory applications. The MAX300-LG is capable of determining all compounds present in a sample, needs minimal sample flow, and provides data updates in a rapid manner (Figure 1).

Figure 1. The MAX300-LG, laboratory gas analyzer, configured for the real-time quantitation of O2, CO2, N2, H2O, and trace volatiles within inspired and expired breath gas samples.
Researchers involved in biomedical or physiological studies of metabolism and respiration can employ breath analysis data to study the underlying processes within complex biological systems (Figure 2).
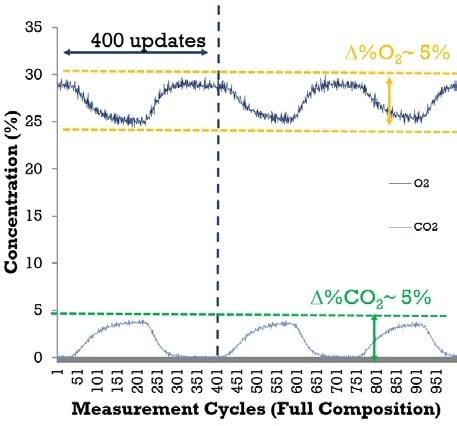
Figure 2. Mass spectrometry for indirect calorimetry, a clinic patient inspiring 30% O2 at a normal breathing rate. The MAX300-LG measures all compounds in the sample with 400 full updates per breath. From this data, metabolic parameters, like the respiratory quotient (RQ) can be calculated without the need for additional equipment to measure flow. O2, and CO2 measurements are shown, RQ=1 ± 0.007 (equation 1).
The MAX300-LG provides high repeatability, which ensures that calculated metabolic parameters obtained from the gas analysis, such as the respiratory quotient (RQ), are precise (Table 1)
Table 1. Typical MAX300-LG breath analysis performance. The mass spectrometer analyzes all compounds in the sample with very high precision. In addition to the components listed here, the analyzer has the flexibility to measure trace volatiles such as formaldehyde, acetic acid, ammonia, and hydrocarbons for the purpose of diagnostic evaluation.
| Name |
Min. Conc. (%) |
Max. Conc. (%) |
Det. Mass |
STD (+/-ppm) |
| Water |
0 |
2.5 |
18 |
54 |
| Nitrogen |
70 |
80 |
28 |
267 |
| Oxygen |
15 |
25 |
32 |
142 |
| Argon |
0.5 |
2 |
40 |
25 |
| Carbon dioxide |
0 |
10 |
44 |
40 |
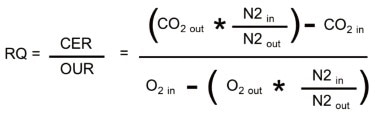
Equation 1. Nitrogen is not absorbed during respiration, so the ratio of the volumetric % of N2 in the inspired and expired sample is used to develop an RQ equation in which measured sample flow is not necessary. All O2, CO2, and N2 terms above are measurements of % volume.
The MAX300-LG’s sample inlet is custom designed for breath analysis application. Present configurations are used for sampling from sub-atmospheric pressures or high pressure (hyperbaric) conditions, like those detected at high altitudes. The inlet is modled to automatically adjust and maintain the peak analytical performance (Figure 3) regardless of the sample pressure.
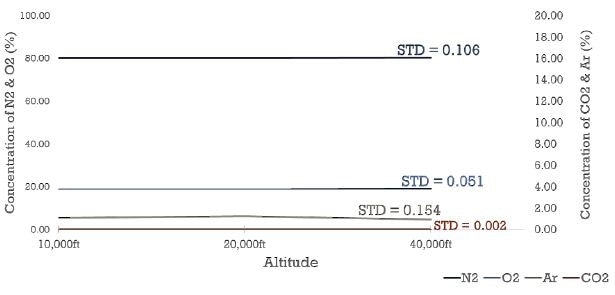
Figure 3. Breath analysis at sub-atmospheric pressures. High altitude conditions were simulated at the MAX300-LG inlet. The system automatically adjusts and delivers uniform, high precision data regardless of sample pressure.
Laboratory gas analyzer, MAX300-LG features a rapid acquisition software and inlet configuration that are custom designed to produce the full breath-to-breath quantitative profile. The gas analyzer provides high precision measurements of all components present in the breath sample, with almost 50 updates for every single compound per second. The complete sample composition enables the scientist to determine metabolic parameters, such as the RQ, without integrating extra flow measurements.
Breath Analyzer Features
The breath analyzer features of the MAX300-LG include:
- Sample transit times as fast as 0.1 seconds
- Measurement rate: up to 5 ms per compound
- Quantitative analysis of O2, CO2, N2, H2O, and trace volatiles
- Minimum sample flow < 0.04 atm cc/s

This information has been sourced, reviewed and adapted from materials provided by Process Insights - Mass Spectrometry.
For more information on this source, please visit Process Insights – Mass Spectrometers.