| Fumed silica has unique properties and is commonly added to liquids/coatings and solids to improve various properties. Adding Fumed Silica to Liquids / Coatings Fumed silica has a chain-like particle morphology. In liquids, the chains bond together via weak hydrogen bonds forming a three dimensional network, trapping liquid and effectively increasing the viscosity. The effect of the fumed silica can be negated by the application of a shear force (e.g. mixing, brushing, spraying etc), allowing the liquid to flow, level out and permit the escape of entrapped air. However, when the force is removed, the liquid will ‘thicken up’ again. This property is called thixotropy. Other useful properties that fumed silica imparts to liquid systems include: • This three dimensional network also helps prevent pigments from settling • When allowed to stand for long enough, coatings become sag resistant • They help to control evaporation in coatings, helping to prevent “picture framing” or “fat edges”, whereby a bead of the coating material forms near the edge of a flat substrate. This phenomena is caused by surface tension effects and faster evaporation near the edges. • Coatings of porous surfaces are also more resistant to absorption by the substrate. • In coatings, it can also aid in pattern development, e.g hammered texture finishes Adding Fumed Silica to Solids / Powders When added to powders, fumed silica aids flow and helps prevent “caking”. The submicron particles are able easily move between larger particles, and some believe it forms a layer on their surface which acts like ball bearings or a lubricant, aiding flow. The hydrophilic nature of the fumed silica also absorbs water off the surface of the particles, preventing caking Production One production method for the production of fumed silica involved a continuous flame hydrolysis technique. It involves the conversion of silicon tetra chloride (SiCl4) to the gas phase using an oxy hydrogen flame. It then reacts with water to yield silica (SiO2) and hydrochloric acid thus: 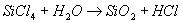
The HCl is easily separated as it remains in the gas phase, while the fumed silica is solid. Treated grades can be produced by reacting the silica fume with organosilicons. This can convert the natural hydrophilic fumed into a hydrophobic material. |