|
Polybutadiene (BR) is the second largest volume synthetic rubber produced, next to styrene-butadiene rubber (SBR). Consumption was about 1,953,000 metric tons worldwide in 1999.
Polybutadiene in Tyres
The major use of polybutadiene is in tyres with over 70% of the polymer produced going into treads and sidewalls. Cured BR imparts excellent abrasion resistance (good tread wear), and low rolling resistance (good fuel economy) due to its low glass transition temperature (Tg). The low Tg, typically <–90C, is a result of the low “vinyl” content of polybutadiene, which will be discussed below. However, low Tg also leads to poor wet traction properties, so polybutadiene is usually blended with other elastomers like natural rubber or styrene-butadiene rubber for tread compounds.
Polybutadiene as an Impact Modifier in Other Polymers
Polybutadiene also has a major application as an impact modifier for polystyrene and acrylonitrile-butadiene-styrene resin (ABS) with about 25% of the total volume going into these applications. Typically about 7% polybutadiene is added to the polymerisation process to make these rubber-toughened resins (see figure 1).
|

|
|
Figure 1. Electron micrograph of a polybutadiene modified or rubber toughened polystyrene resin. Polybutadiene domains are shown in black.
|
Polybutadiene in Golf Balls
Also, about 20,000 metric tons worldwide of “high cis” polybutadiene is used each year in golf ball cores due to its outstanding resiliency. This application is growing since the golf ball industry seems to be moving away from the traditional wound ball technology to the two-piece, solid core construction.
Chemistry and Manufacturing Process
Polybutadiene is a homopolymer (only one monomer) of 1,3 butadiene, a monomer containing four carbon atoms, and six hydrogen atoms (C4H6). The four carbon atoms are in a straight chain containing two “double bonds” as follows:
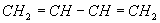
1,3 butadiene
It is the double bonds that are the key to polymer formation. They are attacked by catalysts to maintain a repetitive chain growth process which continues until something is added to terminate the reaction at the desired molecular weight.
Molecular Weight of Polybutadiene
Molecular weight can become quite high. For a typical polybutadiene, molecular weight (Mn = number average) is usually >100,000 grams per mole. This represents a chain that contains over 2,000 butadiene units.
Synthesis of Polubutadiene
Most polybutadienes are made by a solution process, using either a transition metal (Nd, Ni, or Co) complex or an alkyl metal, like butyllithium, as catalyst. Since the reaction is very exothermic, and can be explosive, particularly with alkyllithium catalysts, the reaction is normally carried out in solvents like hexane, cyclohexane, benzene or toluene. The solvents are used to reduce the rate of reaction, control the heat generated by the polymerisation and to lower the viscosity of the polymer solution in the reactor. A typical polybutadiene polymerisation would be run at about 20% monomer and 80% solvent.
Production Using Batch and Continuous Processing
The polymerisation can either be a batch process or a continuous process. In batch mode, monomer, solvent and catalyst are charged to the reactor, heated to initiate the process, and then allowed to continue to completion. The polymer solution is then transferred to another vessel or process unit to remove the solvent. In continuous mode, monomer, solvent and catalyst are continuously fed into the bottom of the first of a series of reactors at a temperature suitable for polymerisation. The polymerisation progresses as the solution flows through the reactors and polymer solution is taken off at the top of the last reactor without stopping the process. The continuous process is the most economical. In both processes, the finished product is usually in the form of bales which weigh from 50 to 75 pounds each.
Types of Polybutadiene
High Cis Polybutadiene
The alkyllithium and transition metal catalysts make very different products. The transition metal, or so called Ziegler catalysts produce very “stereoregular” polybutadienes with one type having the main polymer chain on the same side of the carbon-carbon double bond contained in the polybutadiene backbone. This is called the cis configuration.
|
|
|
Figure 2. Schematic representation of a high cis polybutadiene.
|
High cis polybutadiene will usually have cis content >95% which gives rise to better “green strength” and increased cut growth resistance in the cured product. Green strength, which is the strength of the uncured rubber compound, is important for the tyre building process and cut growth resistance is necessary for tyre performance. Cut growth resistance is the resistance to the propagation of a tear or crack during a dynamic operation like the flexing of a tyre in use. High cis polybutadiene also shows lower Tg compared to alkyllithium-based BR because it has almost no vinyl structure. As mentioned earlier, vinyl tends to increase the Tg of the polymer. The low vinyl content and low Tg makes high cis polybutadiene ideal for golf ball cores. Golf ball cores are cured with peroxides, which tend to “over cure” the vinyl units making a very hard and slow golf ball. The neodymium catalyst system produces the highest cis content of about 99% and also makes the most linear chain structure (no branching) producing a polymer with the best tensile and hysteresis (low heat build-up) properties of all the high cis types. The cobalt system produces a highly branched BR with a low solution viscosity that makes a good polystyrene and acrylonitrile-butadiene-styrene modifier. The nickel catalyst makes polybutadiene with an intermediate level of branching.
|
|
|
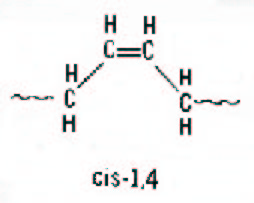
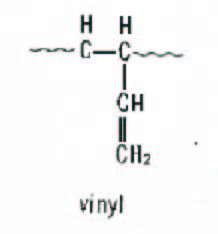
Figure 3. Schematic representation of vinyl.
|
Lithium-based Polybutadiene
The alkyllithium or “anionic” catalyst system produces a polymer with about 40% cis, 50% trans and 10% vinyl when no special polar modifiers are used in the process. The alkyllithium process is probably the most versatile, because the growing chain end contains a “living” anion (negative charge) which can be further reacted with coupling agents or functional groups to make a variety of modified polybutadienes. It also produces gel-free polybutadiene making it ideal for plastics modification. Vinyl increases the Tg of the polybutadiene by creating a stiffer chain structure. Vinyl also tends to crosslink or “cure” under high heat conditions so the high vinyl polymers are less thermally stable than low vinyl. Note above, that in vinyl units the double bonds are pendent to the main chain, giving rise to the special properties of high vinyl polymers. Vinyl units can be increased in lithium-based anionic polymerisation through the use of polar modifiers, which are usually nitrogen or oxygen-containing compounds. The modifiers direct the attack of the propagating anion on the “living” chain end to give a 1,2 addition to the butadiene monomer.
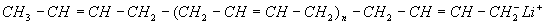
Growing “living” anion (negative charge) on end of live polybutadiene chain with Lithium counterion (positive charge)
High Trans Polybutadiene
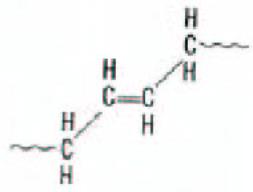
High trans polybutadiene is a crystalline plastic material similar to high trans polyisoprene or balata, which was used in golf ball covers. Note below, that in the trans configuration the main polymer chain is on opposite sides of the internal carbon-carbon double bond. Trans polybutadiene has a melting point of about 80°C. It is made with transition metal catalysts similar to the high cis process (La, Nd, and Ni). These catalysts can make polymers with >90% trans again using the solution process.
|
|
|
Figure 4. Schematic representation of trans 1,4 polybutadiene.
|
Conclusion
Polybutadiene is and will continue to be a high volume rubber for use in tyres, toughened plastics, and golf balls due to its low cost, availability and unique properties. As new markets develop, there will be a need to develop new, higher performance grades of polybutadiene using both the alkyllithium and Ziegler systems.
|