Introduction
Metallocene catalysts have become increasingly important for α-olefin polymerization. The metallocene/methylaluminoxane (MAO) system combines high activity with the possibility of tailoring polymer properties [1]. Depending on the metallocene substituent pattern and symmetry, these catalysts permit a strong control of regio- and stereoregularities and of molecular weight distribution of homopolymers, as well as the synthesis of copolymers with a uniform comonomer distribution. The rapid market penetration of metallocene-based polyethylenes (PE) is due to its high-value attributes, such as greater stiffness and impact strength, greater stretch and puncture resistance and improved sealability. Moreover, polymer properties such as temperature resistance, hardness, impact strength, and transparency can be precisely controlled through the metallocene structure [2].
Unfortunately, these catalysts require a large amount of expensive MAO to reach the maximum catalytic activity, which, to some extent, may impair its value in commercial applications. Moreover, there are other disadvantages in the use of soluble metallocene catalysts. A solution polymerization process requires separation of the polymer and removal, recovery and purification of the solvent. A gas phase process is lower in cost and energy consumption in comparison with the solution process. As most of the existing polymerization plants run a slurry- and gas-phase process with heterogeneous catalysts, the homogeneous catalysts must be heterogenized on a support in order to apply those processes. In addition the heterogenization of metallocene is necessary to avoid reactor fouling with finely dispersed polymer crystals, to prevent excessive swelling of polymers, and to produce polymer particles of a desired regular morphology.
Many routes for the preparation of supported metallocenes have been reported in the literature [3], involving the immobilization directly on bare silica, on MAO-modified silica, among others. In several cases, it is claimed that additional MAO is not necessary during polymerization if it is initially deposited on the surface of silica. These procedures afford different catalysts which, in turn, produce polyolefins with different properties.
The studies involved in heterogenization so far indicate that a simple and physical impregnation or mixing of metallocenes to the support such as silica does not give rise to the formation of a practical catalyst systems mainly due to a drastic reduction of catalytic activity and small quantity of metallocene on the support [4]. Besides, the steric effect played by the silica surface renders difficult the access to the catalyst site, which is also traduced in loss of catalyst activity. One approach to overcome this problem resides in chemically modifying the silica surface with organometallic compounds prior to catalyst grafting. Such compounds play the role of spacers keeping the metallocene catalyst apart from the surface, and therefore more accessible to the olefin.
The preparation of chemically-modified silica can be accomplished, for instance, using alkoxysilane coupling agents [5]. Cyclopentadienyls have been used as ligands for anchoring metallocene complexes on the silica support employing silanes with cyclopentadienyl groups bonded directly to the silicon atom [6]. Iiskola et al. [7] modified a partially dehydroxylated silica surface with a alkoxysilane coupling agent having a hydrocarbon spacer. The catalyst CpIndZrCl2 anchored on silica, which was previously modified with trisiloxane and pentamethylene spacer was shown to be more active than that prepared by reacting the metallocene directly with silica [8]. The immobilization of Cp2ZrMe2 on silica chemically modified with Me3SiCl was claimed to present high activity in ethylene polymerization even in the absence of any cocatalyst [9].
Most of the studies employing chemically-modified silica deal with the use of a spacer between silica surface and metallocene species in order to permit more access of the monomers to the metal active center. We have employed organosilanes as horizontal spacers between zirconocene supported species, aimed at generating catalyst species more spaced among themselves, avoiding eventual bimolecular interactions [10, 11]. In the present work silica was chemically modified by SnCl4 which played the role of horizontal spacer. The supported catalysts prepared by grafting (nBuCp)2ZrCl2 directly on silica and on silica modified by SnCl4 were tested in ethylene homopolymerization having MAO as cocatalyst. Surface metal loadings were determined by Rutherford backscattering spectrometry (RBS) and Zr binding energy (BE) of the surface zirconocene species was measured by X-ray photoelectron spectroscopy (XPS). The influence of Al/Zr ratio and polymerization temperature on catalyst activity and on polymer properties were evaluated. For comparative reasons the homogeneous system was also tested. Polymers were characterized by their molecular weight, crystallinity, polydispersity, melting (Tm) and crystallization (Tc) temperatures.
Experimental
Materials (chemicals)
Silica Grace 948 (255 m2⋅g-1) was activated under vacuum (< 10-2 Pa) for 16 hours at 723 K. The support was then cooled to room temperature under dynamic vacuum and stored under dried argon. MAO (Witco, 10.0 wt.-% toluene solution, average molar mass 900 g⋅mol-1), (nBuCp)2ZrCl2 (Witco) and SnCl4 (Aldrich) were used without further purification. Ethylene, provided by COPESUL Co., and argon were deoxygenated and dried through columns of BTS (BASF) and activated molecular sieve (13 Å) prior to use. Pure grade toluene was deoxygenated and dried by standard techniques before use.
Preparation of supported catalysts
All grafting experiments were performed under inert atmosphere using Schlenk techniques. Typically, an amount corresponding to 1.5 wt.% Zr/SiO2 of (nBuCp)2ZrCl2 toluene solution was added to 1.0 g of activated silica and the suspension was stirred for 6 h at 353 K. The slurry was then filtered through a fritted disk. In the case of the tin-modified silica, the support was previously impregnated with a SnCl4 toluene solution, corresponding to 0.3 wt.% Sn/SiO2, at room temperature for 30 min. The solvent was removed and the metallocene solution was added as described above. The resulting solids were washed with 15 × 2.0 cm3 of toluene and dried under vacuum for 4 hours. The resultant catalysts, (nBuCp)2ZrCl2/SiO2 and (nBuCp)2ZrCl2/Sn/SiO2 presented ca. 0.45 and 0.14 wt.% Zr/SiO2, respectively.
Rutherford backscattering spectrometry (RBS)
Zirconium loadings in catalysts were determined by RBS using He+ beams of 2.0 MeV incident on homogeneous tablets of the compressed (12 MPa) powder of the catalyst systems. The method is based on the determination of the number and the energy of the detected particles which are elastically scattered in the Coulomb field of the atomic nuclei in the target. In this study, the Zr/Si atomic ratio was determined from the heights of the signals corresponding to each of the elements in the spectra and converted to wt.% Zr/SiO2. For an introduction to the method and applications of this technique the reader is referred elsewhere [12].
X-ray photoelectron spectroscopy (XPS)
X-ray photoelectron spectra (XPS) were obtained on a PHI 5600 Esca System (Physical Electronics), using monochromated Al Kα radiation (1486.6 eV). Spectra were taken at room temperature in low resolution (pass energy 235 eV) in the range of 1000 – 0 eV binding energy and in high-resolution (pass energy 23.5 eV) modes for the Si (2p) and Zr (3d5/2) regions. Samples were mounted on an adhesive copper tape. They were prepared in a glove box, transferred under nitrogen atmosphere and then evacuated until reaching 10-4 Pa. During data collection, the ion pumped chamber was maintained at 5 ×10-7 Pa. Takeoff angle (angle between the surface plane and the detector) was 750 in the XPS experiment. Current of the electron gun (neutralizer) was 21.5 mA.
In the case of silica-supported systems, all binding energies values were charge referenced to the silica Si 2p at 103.3 eV. Otherwise, they were referenced to the Au 4f7/2 peak at 84.0 eV. A gold film was previously sputtered on the sample surface in order to get this Au signal. For each of the XPS spectra reported, an attempt was made to fit the experimental curve with a series of peaks that represent the contribution of the photoelectron emission from atoms in different chemical environments. These peaks are described as having Gaussian and Lorentzian contributions in order to take into account the effects of the instrumental error on the characteristic peak shape of the photoemission process.
Polymerization reactions
Ethylene homopolymerizations were performed in 250 cm3 of toluene in a 1.00 dm3 Pyrex glass reactor connected to a constant temperature circulator and equipped with mechanical stirring and inlets for argon and the monomer. MAO was used as cocatalyst in an Al/Zr molar ratio varying from 100 to 5000. For each experiment, a mass of catalyst system corresponding to 10-5 mol of Zr was suspended in 0.01 dm3 of toluene and transferred into the reactor under argon. The polymerizations were performed at atmospheric pressure of ethylene at 318, 333 or 348 K for 30 min. MAO and catalyst were respectively introduced in the reactor containing toluene (1 dm3) under a positive pressure of ethylene. After 30 min., reactions were terminated by shutting off the feed stream, followed by nitrogen purge and polymer precipitation. Acidified (HCl) ethanol was used to quench the processes, and reaction products were separated by filtration, washed with distilled water, and finally dried under reduced pressure at 333 K. Each polymerization reaction was repeated at least 3 times under identical conditions, leading to similar products.
Polyethylene characterization
Polymer melting points (Tm) and crystallinities were determined on a DuPont DSC 2910 differential scanning calorimeter calibrated with Indium, using a heating rate of 10 K⋅min-1 in the temperature range 313 - 513 K. The heating cycle was performed twice, but only the results of the second scan are reported, because the former is influenced by the mechanical and thermal history of the samples. Molar masses and molar mass distributions were investigated with a Waters CV plus 150C high-temperature GPC instrument, equipped with viscometrical detector, optic differential refractometer and three Styragel HT type columns (HT3, HT4 and HT6) with exclusion limit 1 × 107 for polystyrene. 1,2,4 -trichlorobenzene was used as solvent, at a flow rate of 1 cm3⋅min-1. The analyses were performed at 413 K. The columns were calibrated with standard narrow molar mass distribution polystyrene and with linear low density polyethylene and polypropylene.
Results
It is already well-established that a high excess of MAO is necessary to accomplish high polymerization activities when dealing with metallocene catalysts.13 The role of MAO, although not completely elucidated, comprises alkylation of the metallocene, generation of cationic active species and stabilization of these species by coordinative contact with its Cl-MAO- (and / or Me-MAO-) counterion. It is likely that the necessary excess of MAO shifts the reaction equilibrium towards the active species. Moreover, it can convert inactive intermediates into active species by an alkyl exchange reaction [14]. These two effects can account for the number of active species in the medium which in turn afford a high polymerization activity.
Figure 1 shows the trend of homopolymerization activity with different Al / Zr ratios, between 100 and 5000, using MAO as cocatalyst. The highest activities were achieved with the homogeneous system. This fact is totally expected since in this case every catalyst molecule in the reaction medium is potentially an active center. For Al/Zr = 100, the exhibited activity was extremely low. Deffieux et al. [15] demonstrated by UV-Vis spectroscopy study in toluene that it is necessary at least Al/Zr = 150 for the formation of the active metallocene species.
From 100 to 2000 Al/Zr ratio we observe an increase in the catalyst activity. Similar results are reported in the literature for homogeneous metallocene systems [16], in which activity grows with increasing Al/Zr ratios. On the other hand, higher Al/Zr ratios lead to a decrease in activity. The propagation reaction occurs only when the complex formed by the cationic catalyst and MAO is dissociated. Higher amount of MAO might shift the equilibrium towards complexation, thereby reducing the propagation rate.
|
![AZoJomo - Materials Journal Online - Influence of Al / Zr ratio in ethylene homopolymerization activity. Polymerization reactions performed in toluene under 1 atm of ethylene at 333 K with [Zr] = 10-5 mol×L-1](/work/0m893n5eHn691NUEcOxz_files/image001.gif)
|
|
Figure 1. Influence of Al / Zr ratio in ethylene homopolymerization activity. Polymerization reactions performed in toluene under 1 atm of ethylene at 333 K with [Zr] = 10-5 mol⋅L-1
■ (nBuCp)2ZrCl2 ; ●(nBuCp)2ZrCl2/SiO2 ; ▲ (nBuCp)2ZrCl2/Sn/SiO2.
|
The silica-supported system has much lower catalyst activity, in comparison with the homogeneous system, i.e., pure zirconocene. The activities are about six times lower than the corresponding ones of the soluble system. Activity reduction due to catalyst immobilization has already been mentioned in the literature [17] and is attributed to partial destruction of the metallocene by acid centers on the support, besides the generation of some surface species inactive for polymerization. At very low concentrations of MAO, practically no catalyst activity is observed. From 100 to 2000, a fast increase in the polymerization activity was found. Attempts between 2000 and 5000 showed almost the same behavior in polymerization activity.
According to Figure 1, the catalyst activity shown by the tin-modified silica system, although lower than the homogeneous one, presents a much higher activity, in comparison with the (nBuCp)2ZrCl2/SiO2 catalyst. For Al/Zr = 3000, both homogeneous and tin-modified silica catalyst show comparable activities. The employed amount of tin (0.3 wt.-% Sn/SiO2) corresponds to a much lower metal loading than surface saturation [18]. It is worth mentioning that grafting the (nBuCp)2ZrCl2 on silica afforded 0.45 wt.-% Zr/SiO2. Chemical modification with 0.3 wt.-% Sn/SiO2 partially consumed surface silanol groups. Therefore, the resulting grafted zirconocene content is much lower: 0.14 wt.-% Zr/SiO2.
The bimolecular deactivation process is a well-known factor in ethylene and propylene polymerization by zirconocene catalysts [19, 20]. Fischer et al. proposed zirconocene dimers (i.e., electron deficient bridging complexes of two Zr centers) to account for the decay of the polymerization rate [20]. Stehling et al. [21]. suggested that bulky ligand framework would tend to such deactivation. Therefore, grafting (nBuCp)2ZrCl2 on the tin-modified silica may generate a more spaced catalyst species on the surface, which in turn may guarantee a higher stability against bimolecular deactivation. The highly active species derived from the immobilization of the metallocene on tin-modified silica, playing the role of a very bulky ligand framework, appears to suppress the deactivation process by making the mutual approach of Zr centers sterically unfavorable in the bimolecular recombination process.
Iiskola et al. [7] also observed a catalyst activity increase of almost 6-fold/mol Zr when CpZrCl3 was immobilized on a modified-silica surface. In this systems, the support was previously modified with Cp-silicon alkoxyde, which render the catalytic center a little far from the surface. In this case, the high catalyst activity was attributed to an activating effect of the Cp groups on the surface. The direct heterogenization of CpZrCl3 onto unmodified silica produced a catalyst with a very low activity. Lee et al. [8] observed that the catalyst activity of CpIndZrCl2 was 4-5 times higher when the metallocene was supported on trisiloxane or pentamethylene modified silica, in comparison with the CpIndZrCl2/SiO2 system.
The highest activity observed with the tin-modified catalyst may not be attributed to the immobilized zirconocene content. Comparing the Zr content obtained in the case of tin modified-silica with the analogous catalyst prepared directly by grafting on silica, we observe that the metal content is much lower, suggesting that in the case of (nBuCp)2ZrCl2/SiO2 not all the grafted metal content necessarily corresponds to active surface species. Moreover, this behavior is different from that observed with silica modified with trisiloxane or pentamethylene spacer, where the zirconium content is much higher (3.4 wt. %) than that obtained when CpIndZrCl2 is directly supported on silica (0.8 wt.%) [8].
The binding energy (BE) was determined for (nBuCp)2ZrCl2 and both supported systems by XPS. A typical Zr 3d core level spectra is characterized by the presence of two peaks due to spin-orbit coupling of the 3d electrons of Zr: ca. 183 (3d5/2) and 185 eV (3d3/2). Typical zirconocene XPS spectra are shown elsewhere [23, 24].
According to Table 1, grafted systems present Zr atoms bearing a higher BE in comparison to the neat complex. The shift to higher BE indicates the presence of a more electron deficient species, which can be the result of exchange between a chlorine atom from zirconocene and oxygen from silica, the latter being more electronegative. Similar behavior is reported in the literature for Et(Ind)2ZrCl2, which BE shifts from 182.0 eV (neat complex) to 182.7 eV (supported on silica) [24]. Then, the highest catalyst activity exhibited by (nBuCp)2ZrCl2/SnCl4/SiO2 can be partially explained by the presence of more cationic surface species. The correlation of BE and catalyst activity has already been reported in the literature [23,25], and systems bearing higher BE were shown to present higher catalyst activity.
Table 1. Binding Energy of the Zr 3d5/2 core level.
|
|
|
BE (eV)
|
181.6
|
182.4
|
182.6
|
|
FWHM (eV)
|
2.1
|
2.2
|
2.4
|
Concerning polymer characterization, all systems produced PE with melting (Tm) and crystallization (Tc) temperatures practically constant at 408.5 ± 0.5 K and 392 ± 0.8 K, respectively. These melting temperatures are typical of linear high-density polyethylene. Table 2 presents molecular weight (Mw) and polydispersity index (PDI) of the PE produced at different Al/Zr ratios.
According to Table 2, the PEs produced with the two supported catalysts present higher molecular weights comparing to those obtained with the soluble system. This behavior has been already observed in previous results [26], and this fact could be attributed to the blocking of one of the sides of the active site by the support, hindering the deactivation step. In other words, the β-elimination transfer between two metallocene centers is hindered, resulting in a larger growth of the polymer chain, and consequently in a higher molecular weight [27]. It is worth mentioning that polymers with high average molecular weights have better mechanical properties compared to polymers with low average molecular weights.
Table 2. Effect of Al/Zr ratio on the properties of polyethylenes. The polymerization conditions were as follows: P = 1 atm;[Zr]=10-5 mol⋅L-1 (toluene);T = 333 K ; reaction time: 30 min.
|
|
|
100
|
1.4
|
3.3
|
-
|
-
|
-
|
-
|
|
1000
|
3.0
|
2.2
|
3.0
|
2.0
|
2.3
|
1.8
|
|
2000
|
2.7
|
1.8
|
2.7
|
1.8
|
2.0
|
1.8
|
|
3000
|
2.6
|
2.8
|
2.6
|
2.8
|
1.6
|
2.2
|
|
5000
|
2.9
|
2.1
|
2.9
|
2.1
|
2.4
|
2.5
|
The rate of the termination reactions via transfer to a cocatalyst (MAO) should be increased as its concentration increases, which in turn would engender a decrease in molecular weight. This behavior has been reported in the literature [28]. From Table 2, considering the polymers obtained with the soluble system at these operating conditions, Al/Zr ratio of 1000 produces the lowest molecular weight polymer, while of 3000 produces the highest molecular weight polymer. The MWD data (mean of three measures) apparently does not show these trends. Molecular weight seems to be more associated to activity, meaning that the higher the MAO concentration in the medium, the higher the stability of the activity species. Rieger and Janiak [29], also did not observe a significant influence in molecular weight when the amount of MAO is increased in the polymerization. Estrada and Hamielec [30] found only weak evidence that molecular weight decreases with increasing MAO concentration.
For the supported systems, although catalyst activity can vary drastically over time, the MWD produced by those catalysts remains practically unaltered, which indicates that the nature of the active sites is no changed. Between the two supported systems, polymers obtained with the tin-modified support afford lower MWD compared to (nBuCp)2ZrCl2/SiO2 system. It is plausible that the presence of tin derivatives on the surface encourages polymer chain transfer reactions.
The values of PDI ranging from 2.4 to 3.3 are higher than the predicted Flory's most probable distribution (2.0), but they are consistent with the most reported PDIs for soluble single site-type catalysts [31]. PEs produced with both supported systems presented lower PDI than those obtained with the soluble catalyst. It is worth mentioning that polymers with narrow MWD have greater toughness at low temperatures, and higher resistance to environmental stress cracking.
Figure 2 shows the effect of the temperature on the catalyst activity. The reactions were performed at a constant Al/Zr = 2000. Taking into account that for the homogeneous system, the value of ethylene polymerization activity increases sharply as the polymerization temperature increases, and reaches a maximum activity at 333 K. Lower (318 K) or higher (348 K) temperatures lead to a lower activity. Similar trend was already mentioned in the literature [32], using homogeneous ansa-metallocene catalysts.
|
![AZoJomo - Materials Journal Online - Influence of Al/Zr ratio in ethylene homopolymerization activity. Polymerization reactions performed in toluene under 1 atm of ethylene at 333 K with [Zr] = 10-5 mol×L-1](/work/0m893n5eHn691NUEcOxz_files/image002.gif)
|
|
Figure 2. Influence of Al/Zr ratio in ethylene homopolymerization activity. Polymerization reactions performed in toluene under 1 atm of ethylene at 333 K with [Zr] = 10-5 mol⋅L-1
■ (nBuCp)2ZrCl2 ; ●(nBuCp)2ZrCl2/SiO2 ; ▲ (nBuCp)2ZrCl2/Sn/SiO2.
|
It is well-established that the active catalytic species derived from group 4 metallocene precursors is the 14e metallocenium ion produced by a number of cocatalysts, including MAO [33]. A widely accepted mechanism of propagation in Ziegler-Natta catalysis involves the initial formation of a π-complex of an olefin with the transition metal site, followed by chain migratory insertion [34]. At low polymerization temperatures the π-olefin complex is more stable, i.e., its lifetime is greater than the rate of insertion. On the other hand, for high polymerization temperature, the π-olefin complex is unstable and readily dissociates. In other words, the olefin polymerization rate is expected to increase with the temperature in the low region, but the trend is reversed at high temperature. However, the matter may be complicated by the deactivation of catalytic species, because deactivation usually occurs at high temperature, although little is known about the possible chemical transformation of metallocenium species [19]. Thus, the reduction in catalyst activity in the polymerization performed at 348 K could be attributed to a low propagation rate and catalyst deactivation.
A similar trend can be observed with (nBuCp)2ZrCl2/SiO2 system, also reaching its maximum catalytic activity at 333 K, but this variation is larger for the polymerization in homogeneous system than it is in the supported one. A negative effect on the activity was also reported in the copolymerization of ethylene and 1-hexene by Et(Ind)2ZrCl2/MAO systems when the polymerization temperature was raised from 333 to 343 K [35]. It is worth mentioning that attempts to immobilize zirconocene on polysiloxane led to systems which were stable only up to 303 K [36]. It seems that the silica surface stabilizes the active species, affording higher polymerization activities at temperatures close to 333 K.
The (nBuCp)2ZrCl2/Sn/SiO2 systems seem to be less susceptible to the effect of the polymerization temperature. The catalyst activity increases between 318 and 348 K. According to these results, it seems that the catalyst species generated on a tin-modified silica are much more stable than the homogeneous one or than those in the case of the silica. Soga et al. [37] observed an increase in the catalyst activity with increasing the polymerization temperature up to 423 K, using rac-Ph2Si(Ind)2ZrCl2 supported on poly(styrene-co-divinylbenzene) beads.
In Table 3, the effect of polymerization temperature on the molecular weight and on PDI is illustrated. There is a marked reduction of molecular weight for the polyethylene as polymerization temperature increases. Similar behaviors have been mentioned in the literature [27, 35, 36]. The accepted explanation for this widely acknowledged phenomenon is that the propagation and termination rates are both affected by temperature, but termination reactions possess higher activation energies than propagation. Therefore, at lower temperatures, propagation is favored over termination, and the molecular weights become higher. On the other hand, as the temperature increases, activation energy for chain transfer is greater than that for propagation, leading to a decrease in polymer molecular weight.
Table 3. Effect of polymerization temperature on the properties of polyethylenes. The polymerization conditions were as follows: P = 1 atm ; [Zr] = 10-5 mol⋅L-1 (toluene) ; Al/Zr = 2000 ; reaction time: 30 min.
|
|
|
318
|
1.4
|
2.5
|
5.2
|
2.5
|
2.6
|
4.1
|
|
333
|
0.7
|
2.4
|
2.7
|
2.4
|
2.0
|
1.9
|
|
348
|
0.3
|
2.2
|
1.5
|
2.2
|
1.2
|
2.0
|
The PDI seems not to be affected by the polymerization temperature, excluding the case of the reaction performed at 318 K with the tin-modified system. Although catalyst activity can vary drastically over temperature, the PID produced by those catalysts remains practically unaltered, which indicates that the nature of catalyst active sites seems not to be affected.
Conclusion
We demonstrated that it is possible to overcome the problem of low activity of grafted zirconocene catalysts by the previous chemical modification of the silica surface with SnCl4. The reaction between tin-derivative and silica surface partially consume the silanol groups engendering the generation of more spaced zirconocene species, which in turn afford higher catalyst activity. Nevertheless, as shown by XPS analysis, Sn moieties on the surface do not play only the role of a spacer, but influences the chemical nature of the zirconocene species. The higher cationic character might also be responsible for the higher catalyst activity. Besides, such species are thermally more stable. Nevertheless, the resulting polymers present lower molecular weight than those produced with supported zirconocene, grafted on bare silica surface.
Acknowledgments
This work was partially supported by the Brazilian agencies CNPq. Financial support for Prof. J. H. Z. dos Santos has been provided by the Japan Society for Promotion of Science (JSPS).
References
1. B. A. Krentzel, Y. V. Kissin, V. I. Kleiner and L. L. Stotskaya, “Polymers and Copolymers of Higher α-Olefins”, Hanser Publishers, Munich (1997).
2. G. G. Hlatky, “Supported Metallocene Catalysts for Olefin Polymerization” in Metallocene-based polyolefins, (Ed. J. Scheirs and W. Kaminsky), John Wiley & Sons, West Sussex, (2000), Vol. 1, pp. 201-218.
3. G. G. Hlatky, “Heterogeneous Single-Site Catalysts for Olefin Polymerization”, Chem. Rev., 100 (2000) 1347-1376.
4. See, for example: (a) X. Cheng, O. W. Lofthus and P. A. Deck, “Ethylene polymerization using silica-supported zirconocene dibromide/methylalumoxane catalysts”, J. Mol. Catal. A: Chemical, 212 (2004) 121-126; (b) B. Jongsomjit, P. Praserthdam and P. Kaewkrajang, “A comparative study on supporting effect during copolymerization of ethylene/1-olefins with silica-supported zirconocene/MAO catalyst”, Materials Chem. Phys., 86 (2004) 243-246; (c) C. Liu, T. Tang and B. Huang, “Zirconocene catalyst well space inside modified montmorillonite for ethylene polymerization: role of pretreatment and modification of montmorillonite in tailoring polymer properties”, J. Catal., 221 (2004) 162-169; (d) S. Paavola, T. Saarinen, B. Löfgren and P. Pitkänen, “Propylene copolymerization with non-conjugated dienes and a-olefins using supported metallocene catalyst”, Polymer, 45 (2004) 2099-2110.
5. G. L. Witucki, “A Silane Primer; Chemistry and Application of Alkoxy Silanes”, J. Coat. Technol., 65 (1993) 57-67.
6. K. Soga, T. Arai, B.H. Hoang, T. Uozumi, “Olefin polymerization with metallocene catalysts supported on polysiloxane derivaties”, Macromol. Rapid Commun., 16 (1995) 905-911.
7. E. I. Iiskola, S. Timonen, T. T. Pakkanen, P. Lehmus, O. Härkki, J. V. Sappälä,” Functional surface groups for single-site heterogenous α-olefin polymerization catalysts.” Appl. Surf. Sci., 121/122 (1997) 372-377.
8. D. Lee, K., Yoon and S. Noh, “Polymerization of ethylene by using zirconocene catalyst anchored on silica with trisiloxane and pentamethylene spacers”, Macromol. Rapid Commun., 18 (1997) 427-431.
9. B. L. Moroz, N. V. Semikolenova, A. V. Nosov, V. A. Zakharov, S. Nagy and N .J. O'Reilly, “Silica-supported zirconocene catalysts: Preparation, characterization and activity in ethylene polymerization”, J. Mol. Catal A : Chemical, 130 (1998) 121-129.
10. J. H. Z. Dos Santos, P. P. Greco, F. C. Stedile, and J. Dupont, “Organosilicon-modified silicas as support for zirconocene catalyst”, J. Mol. Catal. A: Chemical, 154 (2000) 103-113.
11. M. L. Ferreira, P. P. Greco, J. H. Z. Dos Santos and D.E. Damiani, “A theoretical and experimental study about the effect of different organosilanes on immobilization of (nBuCp)2ZrCl2 on pre-treated SiO2”, J. Mol. Catal. A: Chemical, 172 (2001) 97.
12. (a) F. C. Stedile and J. H. Z. Dos Santos, “Analysis and characterization of real catalysts using ion beam analysis”, Nucl. Instrum. Meth B, 136-138 (1998) 1259-1266; (b) F. C. Stedile, and J. H. Z. Dos Santos, “Characterization of zirconocene catalysts and of polyethylenes produced using them”, Phys. Stat. Sol., (a), 173 (1999) 123-134.
13. See, for example: (a) I. Tritto, S.X. Li, M.C. Sacchi, P. Locatelli, G. Zannoni, Macromolecules, 28 (1995) 5358; (b) D. E. Babushkin, N. V. Semikolenova, V. N. Panchenko, A. P. Sobolev, V. A. Zakharov and E. P. Talsi, “Multinuclear NMR investigation of methylaluminoxane”, Macromol. Chem. Phys., 198 (1997) 3845-3854; (c) I. Tritto, C. Méalares, M. C. Sacchi and P. Locatelli, “Methylaluminoxane: NMR analysis, cryoscopic measurements and cocatalytic ability in ethylene polymerization,” Macromol. Chem. Phys., 198 (1997) 3963-3977; (d) D. Coevoet, H. Cramail and A. Deffieux, “U.V./visible spectroscopic study of the rac-Et(Ind)2ZrCl2/MAO olefin polymerization catalytic system, 2. Investigation in CH2Cl2”, Macromol. Chem. Phys., 199 (1998) 1459-1464.
14. W. Kaminsky, “How to Reduce the Ratio MAO/Metallocene”, Macromol. Symp., 97 (1995) 79-89.
15. D. Coevoet, H. Cramail and A. Deffieux, “U.V./visible spectroscopic study of the rac-Et(Ind)2ZrCl2/MAO olefin polymerization catalytic system, 1. Investigation in toluene”, Macromol. Chem. Phys., 199 (1998) 1451-1457.
16. F. Forlini, Z.-Q. Fan, I. Tritto, P. Locatelli and M.C. Sacchi, “Metallocene-catalyzed propene/1-hexene copolymerization: Influence of amount and bulkiness of cocatalyst and of solvent polarity”, Macromol. Chem. Phys., 198 (1997) 2397-2408.
17. W. Kaminsky, “New polymers by metallocene catalysis”, Macromol. Chem. Phys., 197 (1996) 3907-3945.
18. D. Ballivet-Tkatchenko, J. H Z. Dos Santos and M. Malisova, “Reactions of organostannanes with silica, γ-alumina, and silica-alumina”, Langmuir, 9 (1993) 3513-3517.
19. J. C. W. Chien and B. P. Wang, “Metallocene-methylaluminoxane catalysts for olefin polymerization. V. Comparison of Cp2ZrCl2 and CpZrCl3” J. Polym. Sci. A: Polym. Chem., 28 (1990) 15-38.
20. (a) D. Fischer and R. Mulhaupt, “Reversible and irreversible deactivation of propene polymerization using homogeneous Cp2ZrCl2/methylaluminoxane Ziegler-Natta catalysts, J. Organomet. Chem., 417 (1991) C7-C11; (b) D. Fischer, S. Jungling and R. Mulhaupt, “Donor and acceptor-modified metallocene-based homogeneous Ziegler-Natta catalysts”, Makromol. Chem., Macromol. Symp., 66 (1993) 191-202.
21. U. Stehling, J. Diebold, R. Kirsten, W. Röll, H. H. Brintzinger, S. Jüngling, R.Mülhaupt and F. Langhauser,” Ansa-Zirconocene Polymerization Catalysts with Anellated Ring Ligands - Effects on Catalytic Activity and Polymer Chain Length”, Organometallics, 13 (1994) 964-970.
22. M. C. Haag, C. Krug, J. Dupont, G. B. Galland, J. H. Z. Dos Santos, T. Uozumi, T. Sano and K. Soga, “Effects of Al/Zr ratio on ethylene-propylene copolymerization with supported-zirconocene catalysts”, J. Mol. Catal. A: Chemical, 169 (2001) 275-287.
23. J. H. Z. Dos Santos, H. T. Ban, T. Teranishi, T. Uozumi, T. Sano and K. Soga, “Supported metallocenes using inorganic-organic hybrid xerogels”, J. Mol. Catal. A: Chemical, 158 (2000) 541-557.
24. M. Atiquilah, F. Faiz, M. N. Akhtar, M. A. Salim, S. Ahmed and J. H. Khan, “XPS Investigation of the electronic environment in selected heterogenized zirconocene catalysts”, Surf. Interface Anal., 27 (1999) 728-734.
25. P. G. Gassman and M. R. Callstrom, “Isolation, and partial characterization by XPS, of two distinct catalysts in the Ziegler-Natta polymerization of ethylene”, J. Am. Chem. Soc., 109 (1987) 7875-7876.
26. J. H. Z. Dos Santos, S. Dorneles, F. C. Stedile, I .J. R. Baumvol, J. Dupont and M. M. C. Forte, “Silica supported zirconocenes and Al-based cocatalysts: surface metal loading and catalytic activity”, Macromol. Chem. Phys., 198 (1997) 3529-3537.
27. W. Kaminsky and F. Renner,”High melting Polypropenes by Silica-supported Zirconocene Catalysts”, Makromol. Chem., Rapid Commun., 14 (1993) 239-248.
28. L. D'Agnillo, J. B. P. Soares and A. Penlidis, “Effect of operating conditions on the molecular weight distribution of polyethylene synthesized by soluble metallocene/methylaluminoxane catalysts”, Macromol. Chem. Phys., 199 (1998) 955-962.
29. B. Rieger and C. Janiak, “Silica Gel Supported Zirconocene Dichloride/Methylalumoxane for Ethylene Polymerizations: Effects of Heterogenation on Activity, Polymer Microstructure and Product Morphology”, Angew. Makromol. Chem., 215 (1996) 45-57.
30. J. M. V. Estrada and A. E. Hamielec, “Modelling of ethylene polymerization with Cp2ZrCl2/MAO catalyst”, Polymer, 35 (1994) 808-818.
31. See, for example: (a) R. Quijada, J. Dupont, D. C. Silveira, M. S. L. Miranda and R. B. Scipioni, “The influence of the transition metal and the heteroatomic-bridge on the action of metallocene/methyl aluminoxane catalysts in ethylene polymerization and on the properties of the polymer”, Macromol. Rapid Commun., 16 (1995) 357-362.
32. Y. Chen, M. D. Rausch and J. C. W. Chien, “Heptane-soluble homogeneous zirconocene catalyst: Synthesis of a single diastereomer, polymerization catalysis, and effect of silica supports”, J. Polym. Sci. A: Polym. Chem., 33 (1995) 2093-2108
33. See, for example: (a) J. C. W. Chien, W.-M. Tsai and M. D. Rausch, “Isospecific polymerization of propylene catalyzed by rac-ethylenebis(indenyl)methylzirconium cation”, J. Am. Chem. Soc., 113 (1991) 8570-8571; (b) W.-M. Tsai, M. D. Rausch and J. C. W. Chien, “Low-temperature isospecific polymerization of propylene catalyzed by alkylzirconocene-type 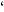 cations cations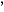 “, Appl. Organomet. Chem., 7 (1993) 71-74; (c) R. F. Jordan,”Cationic metal alkyl olefin polymerization catalysts”, J. Chem., Ed. 65 (1998) 285-289; (d) C. Sishta, R. M. Hathorn and T. J. Marks, “Group 4 metallocene-alumoxane olefin polymerization catalysts. CPMAS-NMR spectroscopic observation of cation-like zirconocene alkyls”, J. Am. Chem. Soc., 114 (1992) 1112-1114. “, Appl. Organomet. Chem., 7 (1993) 71-74; (c) R. F. Jordan,”Cationic metal alkyl olefin polymerization catalysts”, J. Chem., Ed. 65 (1998) 285-289; (d) C. Sishta, R. M. Hathorn and T. J. Marks, “Group 4 metallocene-alumoxane olefin polymerization catalysts. CPMAS-NMR spectroscopic observation of cation-like zirconocene alkyls”, J. Am. Chem. Soc., 114 (1992) 1112-1114.
34. P. Cossee, “Ziegler-Natta catalysis I. Mechanism of polymerization of α-olefins with Ziegler-Natta catalysts”, J. Catal., 3 (1964) 80-88.
35. S. K. Noh, S. Kim, J. Kim, D.-H. Lee, K.-B. Yoon, H.-B. Lee, S. W. Lee and W. S. Huh, “Investigation of the polymerization behavior of polysiloxane-bridged dinuclear zirconocenes as model compounds for a heterogenized metallocene at the silica surface”, J. Polym. Sci. A : Polym. Chem., 35 (1997) 3717-3728.
36. M. Eskelinen and J.V. Seppälä, “Effect of polymerization temperature on the polymerization of ethylene with dicyclopentadienylzirconiumdichloride/ methylalumoxane catalyst”, Eur. Polym., J. 32 (1996) 331-335.
37. S. C. Houg, H. T. Ban, N. Kishi, J. Jim, T. Uozumi and K. Soga ,”Ethene polymerization with a poly(styrene-co- divinylbenzene) beads supported rac-Ph2Si(Ind)2ZrCl2 catalyst”, Macromol. Chem. Phys., 199 (1998) 1393-1397.
Contact Details
|