Sep 25 2001
Background
Dumas predicted the existence of silicone rubbers ion 1840. however, it was not until 1872, that Ladenburg produced the first example, a very viscous oil, by reacting diethoxydiethysilane with water and trace amounts of acids. The first commercial grades were produced in 1943 by the Dow Chemical Company, with many other companies following shortly after.
Structure and Chemistry
Silanes
Silanes are analogous to hydrocarbons, i.e, the basic building block of hydrocarbons is the CH4 (methane) group, while that for silianes is SiH4 (silane) and the basic structure is SiH3(SiH2)nSiH3. These materials have the Si-Si-Si backbone and the resultant structures are named based on the number of silicon atoms in the chain i.e. silane, disilane, trisilane, tetrasilane etc etc. Furthermore, similar substitutions can take place, as is the case with hydrocarbons, e.g. CH3 groups for SiH3 groups, Cl for H, etc. Naming conventions are the same as for the hydrocarbon analogues.
Siloxanes
Siloxanes differ from silanes in that they have a Si-O-Si backbone and a general formula SiH3(OSiH2)nOSiH3. Naming conventions are similar to silanes i.e. disiloxane, trisiloxane etc.
Those materials having mainly organic side groups attached to silicon atoms are referred to as polyorganosiloxanes of silicones.
Classes of Silicone Rubbers
According to ASTM D1418 there are various classes of silicone rubbers outlined in table 1.
Table 1. ASTM D1418 Silicone rubber classifications.
| Class |
Description |
Application |
| MQ |
Silicone rubbers having only methyl groups on the polymer chain (polydimethyl siloxanes) |
Not commonly used |
| VMQ |
Silicone rubbers having methyl and vinyl substitutions on the polymer chain |
General purpose |
| PMQ |
Silicone rubbers having methyl and phenyl substitutions on the polymer chain |
Extremely low temperature applications
Not commonly used |
| PVMQ |
Silicone rubbers having methyl, phenyl and vinyl substitutions on the polymer chain |
Extremely low temperature applications |
| FVMQ |
Silicone rubbers having fluoro, methyl and vinyl substitutions on the polymer chain |
Applications involving fuel, oil and solvent resistance. |
Industrial Classifications
There are three main industrial classifications of silicone rubbers:
• High Temperature Vulcanising (HTV) – Sometimes called heat curable, these are usually in a semi-solid gum form in the uncured state. They require rubber-type processing to produce finished items.
• Room Temperature Vulcanising (RTV) – Usually come as a flowable liquid and are used for sealants, mould making, encapsulation and potting. These materials are not generally used as conventional rubbers.
• Liquid Silicone Rubbers (LSR) – Sometimes called heat curable liquid materials, these materials are processed on specially designed injection moulding and extrusion production equipment.
Synthesis of Silicones
The most common method for preparing silicones involves reacting a chlorosilane with water. This produces a hydroxyl intermediate, which condenses to form a polymer-type structure. The basic reaction sequence is represented as:
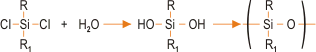
This is the favoured route although other raw materials such as alkoxysilanes can be used. Chlorosilanes and other silicone precursors are synthesised using the “Direct Process”, involving the reaction of elemental silicone with an alkyl halide thus,
Si + RX → RnSiX4-n (where n = 0-4)
Preparation of silicone elastomers requires the formation of high molecular weight (generally greater than 500000g/mol). To produce these types of materials requires di-functional precursors, which form linear polymer structures. Mono and tri-functional precursors form terminal structures and branched structures respectively.
Other Components in Silicones
Curing Additives
With the exception of RTV and liquid curing systems, silicone rubbers are usually cured using peroxides such as benzoyl peroxide, 2,4-dichlorobenzoyl peroxide, t-butyl perbenzoate and dicumyl peroxide. Alkyl hydroperoxides and dialkyl peroxides have also been used successfully with vinyl containing silicones.
Hydrosilylation or hydrosilation is an alternative curing method for vinyl containing silicones and utilises hydrosilane materials and platinum containing compounds for catalysts. It is a 2-part process requiring mixing of 2 separate components, with the resulting material having a limited shelf life. Curing does not produce volatiles and heat cured conventional silicones with high tear strengths can be cured in this way.
Fillers
Reinforcing fillers are added to improve the otherwise poor tensile strength of silicones. Silica, in the form of silica fume with particle sizes in the range 10-40nm is the most preferred filler, although carbon black has been used. Fillers do interact with the vulcanisate, forming a pseudo-vulcanisation. This can occur either during mixing (creep hardening) or in storage (bin ageing).
Although milling can break down these structures, it is also common to add structure control additives or ant-structure additives to combat these reactions. Examples of these materials are siloxane-based materials such as diphenylsilane and pinacoxydimethylsilane.
Other Additives
Silicones have better fire resistant properties compared to natural rubbers. This property can be improved by the addition flame retardant additives such as platinum compounds, carbon black, aluminium trihydrate, zinc or ceric compounds. It should be noted that carbon black addition also increase electrical conductivity.
Ferric oxisde may also be added to improve heat stability, titanium dioxide and other organometallic compounds as pigments.
Manufacture
Silicones can be mixed/compounded using mixers of mills. However, due to the low viscosity close-fitting scrapers and cheek plates need to be used to ensure complete mixing. Forming can be carried out by conventional techniques such as injection moulding, extrusion and compression moulding. Care must be taken to take into account relatively large curing shrinkages and to avoid entrapped air.
Curing is generally rapid for most grades and folowed by a post cure treatment in an air oven at 200-250°C, for a period of 4-24 hours. This process serves to improve properties and remove residual peroxide products.
Liquid Silicone Rubbers
These are essentially two-part systems, supplied deaerated ready for use often in premetered equipment. Low injection pressures and low pressure forming techniques are sufficient. They cure after mixing the two separate portions, by processes such as hydrosilylation. Curing is often complete in as little as a few seconds at temperatures of about 200°C and post-curing is not usually required.
The low capital investment required for production mean that LSRs can compete with conventional silicones and organic rubbers.
Physical properties are comparable to general purpose grades and high strength peroxide cured elastomers. Furthermore, they exhibit self-extinguishing properties, with carbon black additions enabling them to satisfy UL-94 tests.
Room Temperature Vulcanising (RTV) Rubbers
These are available in one (RTV-1) and two-part (RTV-2) systems.
Single part systems consist of polydialkylsiloxane with terminal hydroxyl groups, which are reacted with organosilicon cross-linking agents. This operation is carried out in a moisture-free environment and results in the formation of a tetrafunctional structure. Curing takes place when materials are exposed to moisture. Atmospheric moisture is sufficient to trigger the reaction, and thickness should be limited if only one side is exposed to the moisture source. Curing is also relatively slow, reliant on moisture ingress into the polymer.
Two pack systems can be divided into two categories, condensation cross-linked materials and addition cross-linked polymers.
Condensation systems involve the reaction of silanol-terminated polydimethylsiloxanes with organosilicon cross-linking agents such as Si(RO)4. Storage life depends on the catalyst employed and ambient conditions.
Addition-cured materials must be processed under clean conditions as curing can be affected by contaminants such as solvents and catalysts used in condensation RTVs. These materials are suited to use with polyurethane casting materials.
Key Properties
Advantages
Properties that have made this family of rubbers important engineering materials include:
• Good thermal stability
• Constancy of properties over a wide temperature range leading to large operating range (e.g. –100 to 250°C)
• Ability to repel water and form water tight seals
• Excellent resistance to oxygen, ozone and sunlight
• Flexibility
• Good electrical insulation
• Anti-adhesive properties
• Low chemical reactivity
• Low toxicity
Disadvantages
• Vulcanised rubbers display poor tensile properties
• Some grades have poor hydrocarbon, oil and solvent resistance
• High gas permeability (not always a problem)
• Relatively high cost
Thermal Stability
The thermal stability of silicones stems from the thermal stability of Si-O and Si-CH3 bonds which are themselves thermally stable. However, the partially ionic nature of these bonds (51%), means that they can be easily destroyed by concentrated acids and alkalis at ambient temperatures.
Flexibility
In general these materials are flexible at low temperatures due to their low glass transition temperature (Tg). However, they also tend to stiffen up at higher temperatures.
Resistance to Hydrocarbons, Oils and Solvents
The first compositions to exhibit oil resistance were those that had nitrile groups (CN) (figure 1) substituting for some of the methyl groups. These were superseded by silicones containing fluorine, which display excellent resistance to oils, hydrocarbons and solvents.
|
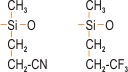
|
|
Figure 1. Oil resistant grades of silicone rubber with (left) nitrile functional groups and (right) fluorine functional groups.
|
Gas Permeability
At 25°C the permeability of silicone rubber is approximately 400 times that of butyl rubber. This allows this material to be used for gas permeable applications such as oxygen permeable membranes in medical applications.
Electrical Properties
Silicones are excellent electrical insulators with grades available with volume resistivities as low as 0.004 ohm.cm. Their thermal stability means that properties such as volume resistivity, dielectric strength and power factor are not affected my changes in temperature. They also display arc and corona resistances surpassed only by mica.
Applications
Mechanical Engineering
Examples of mechanical engineering applications include:
• Shaft sealing rings
• Spark plug caps
• Radiator and automotive heating hoses
• O-rings
• Corona and embossing roller gaskets
• Window and door seals
• Expansion Joints
Electrical Engineering
Examples of electrical engineering applications include:
• Cables and cable terminations
• Corona-resistant insulation tubing
• Keyboards and contact mats
• Conductive profiled seals
Medical
Examples of medical applications include:
• Tubing for dialysis and transfusion equipment
• Bellows for artificial respirators
• Catheters
• Dummies for babies
Primary author: AZoM.com