Potency determination by PotencyMR has been proved to be a single point replacement for regular development testing which earlier involved several experiments and techniques1, including potency determination, relative response factor calculation, moisture analysis, residual solvent, identity testing, absolute compound purity assessment.
It is also a better method for establishing the purity of the reference standards2 used for analysis with guaranteed metrological traceability3 such as standards for HPLC.
The disadvantage is that existing PotencyMR workflows rely on well-trained analysts and/or professional knowledge and detailed protocols.
The new potency determination tools from Bruker streamline this process with a completely automatic workflow from sample submission to report, making it a perfect solution for both non-experts and experts working in a pharmaceutical development environment, where quality is essential.

Technical Details
Potency by NMR - Your Benefits
Faster: NMR is inherently quantitative. There is no need to calculate calibration curves or response factors.
Versatile: a completely characterized reference standard is not needed for the analyte. Commercially available reference standards are used.
Accurate: use of internal standard prevents errors caused by inherent sample differences.
Reproducible: an automated workflow from acquisition to analysis decreases variability and human error.
Flexible and intuitive: straightforward manual interaction when desired.
All-in-one: structure and potency confirmation in one single experiment. Inorganic and organic materials are considered. Use of additional techniques is not needed.
Key Features
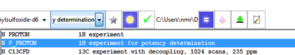
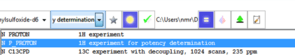
Straightforward sample submission through IconNMR automation software. For most commonly used internal standards, optimal qNMR experiments are also provided as parameters.
Automated data analysis comprises of internal standard peak identification and integration with sophisticated ‘peak snapping’ algorithm, potency calculation, consistency analysis, and analyte quantification. Modification of the workflow according to SOPs which define the analyte's integral regions.
Error analysis within the sample: multiple analyte peaks are integrated, averaged and the RSD is given.
Error analysis between samples: replicate samples can be submitted with just one click. Potency for each replicate will be calculated. Final averaged result and associate error are provided.
Results are presented in varied formats such as PDF report with excel table output and spectral information.
The automatic data analysis is based on Bruker’s proven algorithms for NMR quantification. These can either be manually executed or are automatically called from the acquisition module. In addition to the automatic analysis, it is possible to access all processing features and the subsequent analysis for manual work in an easy-to-use manner.
References
1. Webster G.F. and Kumar S., Anal Chem, 86, 11474 (2014)
2. Pauli G.F., Godecke T., Jaki B.U. and Lankin D.C., J Nat Prod, 75, 834 (2012)
3. Japanese Pharmacopeia XVII, General Tests and Apparatus, p. 137