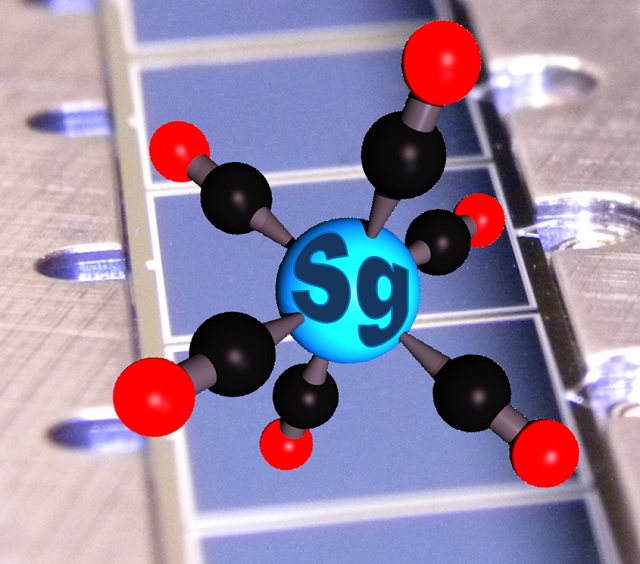
 This is a graphic representation of a seaborgium hexacarbonyl molecule on the silicon dioxide covered detectors of a COMPACT detector array.
This is a graphic representation of a seaborgium hexacarbonyl molecule on the silicon dioxide covered detectors of a COMPACT detector array.
A group of researchers from Mainz and Darmstadt have established a chemical bond between a carbon atom and a superheavy element for the first time ever. The researchers established a chemical bond between seaborgium and a carbon atom by converting eighteen atoms of seaborgium into seaborgium hexacarbonyl complexes.
The Superheavy Element team working at Japan’s RIKEN Nishina Center focused on seaborgium production using the fusion of a neon beam using a curium target and achieved its isolation in the GAs-filled recoil ion separator (GARIS).
In the conventional technique for producing superheavy elements, large amounts of byproducts often disturb the detection of single atoms of superheavy elements such as seaborgium. Using the GARIS separator, we were able at last to catch the signals of seaborgium and evaluate its production rates and decay properties. With GARIS, seaborgium became ready for next-generation chemical studies.
Dr. Hiromitsu Haba, team leader at RIKEN
The teams combined their individual research from 2013 and managed to successfully detect 18 seaborgium atoms. When studied closely, it was found that the gaseous properties and the adsorption on a silicon dioxide surface were similar to those of the corresponding hexacarbonyls of molybdenum and tungsten, which belong to group 6 of the periodic table, thereby proving that the detected compound is seaborgium hexacarbonyl.
This study will be useful in order to examine relativistic effects on the characteristics of heavy chemical elements. The method used by the researchers will enable studies on the chemical properties of single, short-lived atoms as well as less stable compounds.
The study also opens doors for the advanced investigation of superheavy elements in more detail. Going forward, the team plans to continue their research into other compounds which are heavier than seaborgium.