Sep 29 2014
Researchers at Berkeley Lab have used bimetallic nanoparticles as a catalyst in the process for the reduction of carbon dioxide. Chemist Peidong Yang of the Materials Sciences Division at Berkeley Lab has led the study.
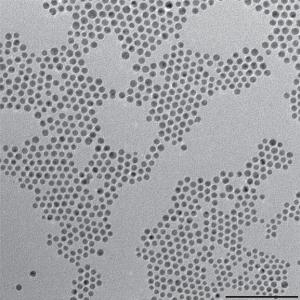 This TEM shows gold-copper bimetallic nanoparticles used as catalysts for the reduction of carbon dioxide, a key reaction for artificial photosynthesis.Credit: Image courtesy of Peidong Yang group, Berkeley Lab
This TEM shows gold-copper bimetallic nanoparticles used as catalysts for the reduction of carbon dioxide, a key reaction for artificial photosynthesis.Credit: Image courtesy of Peidong Yang group, Berkeley Lab
Artificial photosynthesis could be used for production of sustainable, green fuels. This process involves reduction of carbon dioxide electrochemically. However, processes require a suitable catalyst and finding an efficient catalyst with the required properties has been challenging, till now. Global climate change is caused by abundant atmospheric carbon dioxide that is more than necessary. The environment, as well as the economy could achieve significant benefits if this excess could be converted and used as energy. Artificial photosynthesis holds promise for this conversion.
The researchers at Berkeley discovered that geometric and electronic effects synergistically played an important role in carbon dioxide reduction reaction. They control the binding strength of the reaction intermediates, which influences the efficiency and selectivity of the catalyst in the reduction process.
Yang used nanocatalysts made from metal alloys for this process instead of single metals. Alloying helped improve the reaction kinetics for the reduction of carbon dioxide by tuning the binding strength of intermediates that are on the surface of the catalyst. Usage of nanoparticles in the study enabled precise control of specific sites and provided better analysis. Further, nanoparticle catalysts possessed high and surface-to-mass and surface-to-volume ratios that improved catalytic activity.
The researchers assembled gold–copper bimetallic nanoparticles with varying compositions into ordered monolayers, which enabled better analysis of the catalytic activity in reduction reaction.
The effects observed in the study could hold good for other catalysts in achieving exceptional advancements in electrochemical carbon dioxide reduction.
References