Dec 5 2014
Researchers at the Pacific Northwest National Laboratory have developed a rubber-like coating that can increase the durability of high capacity silicon electrodes for creating rechargeable lithium batteries with higher-capacity and longer life.
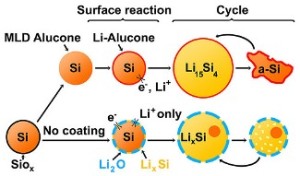 Silicon nanoparticles coated in alucone (yellow spheres outlined in orange) expand and contract easily on charging and use. But left to their native silicon oxide covering (yellow spheres in blue), they break down fast on recharging.
Silicon nanoparticles coated in alucone (yellow spheres outlined in orange) expand and contract easily on charging and use. But left to their native silicon oxide covering (yellow spheres in blue), they break down fast on recharging.
Traditionally, graphite electrodes have been used in rechargeable lithium batteries. However, they have a low capacity for which silicon electrodes hold promise as a potential replacement.
Silicon electrodes have a high electrical capacity and ten-times more capacity than graphite electrodes, which results in a longer life. However, they have very low durability. They lose their capacity to hold electricity after many charge cycles.
Silicon absorbs lithium like a sponge. Lithium penetrates the silicon electrode during charging and this causes the silicon electrode to swell up to three times its original size. However, silicon breaks down and fractures. In order to address these shortcomings, the researchers used 150nm wide silicon spheres. This tiny size enabled rapid and thorough recharge.
Earlier studies had discovered that silicon nanoparticles coated with aluminum glycerol had five times longer life (more than 150 cycles). The nanoparticles grew on a hard silicon oxide shell. However, it was not understood how the coating improved the electrode’s performance.
A special instrument at EMSL was used to study the coated silicon nanoparticles when they were active. When the silicon nanoparticles did not have the alucone coating, the silicon was prevented from expanding by the oxide shell, which in turn limited the intake of lithium by the particles.
The alucone coating softened the silicon nanoparticles and facilitated easier expansion and shrinking.
The researchers observed that during charging, particles having oxide shells demonstrated a tendency to merge together and prevent lithium from penetrating the silicon. The rubbery alucone coating enabled optimal performance by keeping the particles separated.
This study has been published in the journal, ACS Nano.