Sep 14 2015
Researchers at the Max-Planck-Institut für Eisenforschung in Düsseldorf have discovered that manganese steel forms a different crystal structure at typical linear defects.
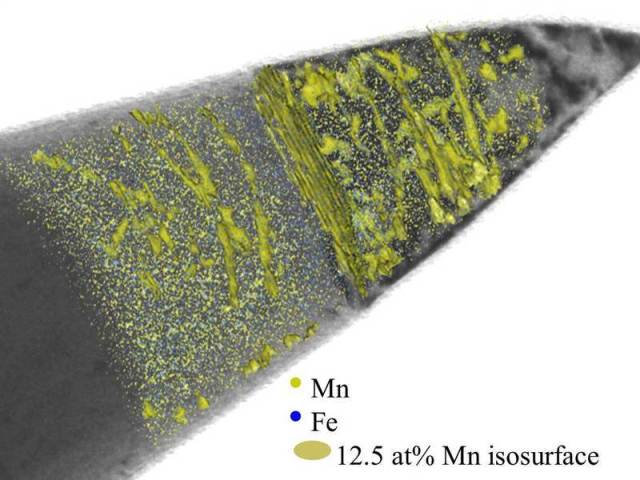 Structural change in steel: Scientists at the Max-Planck-Institut für Eisenforschung use images from a transmission electron microscope (grey) to make linear defects in an alloy of iron (Fe) and manganese (Mn) visible. Atom probe tomography shows them the distribution of the iron (blue) and manganese atoms (green). They have put green iso-surfaces into the image where the concentration of the manganese atoms is 12.5 percent. In the superimposed images, the researchers can see that the manganese atoms accumulate along the linear defects; the crystal structure which forms there is different to the surrounding material. © M. Kuzmina/MPI für Eisenforschung
Structural change in steel: Scientists at the Max-Planck-Institut für Eisenforschung use images from a transmission electron microscope (grey) to make linear defects in an alloy of iron (Fe) and manganese (Mn) visible. Atom probe tomography shows them the distribution of the iron (blue) and manganese atoms (green). They have put green iso-surfaces into the image where the concentration of the manganese atoms is 12.5 percent. In the superimposed images, the researchers can see that the manganese atoms accumulate along the linear defects; the crystal structure which forms there is different to the surrounding material. © M. Kuzmina/MPI für Eisenforschung
The current discovery about manganese steel may be considered to provide both good and bad properties. At typical linear defects, different crystal structure is formed. Metals are made up of individual crystal grains and these can be considered to be like a stack made up of individual atomic layers. Edge dislocations can take place when a specific layer remains incomplete, and the layers that are above and below this layer have to take a step.
In one cubic metre of steel, the length of the linear defects in it could be equivalent to a light year. The structure of steel is dependent upon many properties, including rigidity, malleability, and ductility, and this discovery could aid scientists endeavouring to optimize the alloy.
Lives can be saved through dislocations due to the key role played by 1D defects of a metal in its deformation. During an accident, the body panel of a vehicle crumples to absorb most of the impact energy, and this process could prevent the passengers from getting injured. The metal bends at dislocations, which act as nano-hinges. The manner of deformation of the metal would also be affected by how the crystal structure is different from the structure that is around the linear defect.
In extreme cases, the metal may tear down instead of deforming. “We don’t yet know what effect the spatially confined chemical and structural states in the material have on its properties,” says Dierk Raabe, Director at the Max-Planck-Institut für Eisenforschung and head of the study in which the deviationists in the microstructure have just come to light.
“We stumbled across the states more by chance,” says Dierk Raabe. His research team has been studying a specific manganese steel that is quite rigid and ductile. Nanoparticles are used to strengthen this steel, and this material is typically used in large aircraft for their landing gear applications. The researchers studied the micro- and nano-structure of this material using atom probe tomography. In this process, short electric voltage pulses are used to vaporize a sample atom by atom. The specific element to which the vaporized detached atom belongs can be determined from the time-of-flight of the atom to a detector. The specific location where the atom hits the detector can help find out its position.
“We noticed that the concentration of the manganese increased along specific lines after we had heated the material,” explains Dirk Ponge, an important contributor to the study. The team observed that the manganese collects in 2nm wide tubes, and in the form of a manganese-rich nano-bead chain.
The material’s crystal structure must change in order to contain the comparatively larger number of manganese atoms in these very small areas. In a cubic unit cell, typically, manganese and iron atoms sit at the centres and the corners. This is considered to be a martensite or body-centred cubic structure. In the nano-bead chain, the manganese concentration corresponds to an arrangement called austenite or face-centred cubic structure.
Earlier, such deviations were believed to exist in a metal’s regular crystal structure in a 2D form. However, in this case the material scientists found filigree austenite structures in the inner part of the individual martensite crystal grains. “When we saw that the manganese accumulated in thin tubes, we had the idea there could be spatially confined chemical and structural states along linear defects,” says Dirk Ponge.
To confirm this, Ponge and his colleagues used a transmission electron microscope to scan an iron-manganese sample. This revealed the linear defects. They then used atom probe tomography to map the atom distribution in the sample. When they superimposed images obtained from both these methods, they discovered the manner in which the manganese-rich nano-beads arranged themselves accurately along the linear defects.
The researchers explained the reason why the atoms arranged themselves accurately along the dislocations in a manner that was different from the remaining crystals. “The stress is particularly high at the dislocations,” says Dirk Ponge. “The material can apparently reduce stress and thus assume an energetically more favourable state by forming a crystal structure there which would be energetically less favourable otherwise.”
The Düsseldorf-based researchers then extended an important formula used for calculating the structure that a material prefers to stay under specific conditions where such structural defects exist.
The team utilized heat to mobilize the atoms so that they took on a more energetically favourable structure only at the dislocation area. “This doesn’t mean that the spatially confined chemical and structural states form only when heat is applied, however,” says Dierk Raabe.
Such material states can also be found in places apart from turbine blades, motor’s cylinders and other materials that are always under the influence of high heat conditions. “Small atoms, such as those of carbon, are much more mobile than those of manganese,” explains Dierk Raabe. “We therefore must assume that we will find the spatially confined states in carbon containing car body steel panels as well.”
Further on, the researchers want to study the effect of the local structural change on the material’s properties. “Our findings may help to explain an already known behaviour of metals – the fact, for instance, that metals become brittle when they corrode and absorb hydrogen,” says Dierk Raabe.
The team states that the fact that the crystal structure at linear defects moves out of line may not be bad all the time. “Maybe we can bring about these spatially confined states intentionally in order to develop a nano-Damascus steel that forges itself,” says the Max Planck Director.
Damascus steel is so named as it came through Damascus to Europe. In the Orient, expert craftsmen had forged a ductile, hard steel that was also soft and brittle into a hard composite material that is difficult to break. Further research may help find a simple method to combine these numerous material properties that are incompatible with each other, and dislocations may help provide such a desired structure. Such a discovery could offer new ways for the steel industry to optimize materials for desired applications.