Aug 18 2016
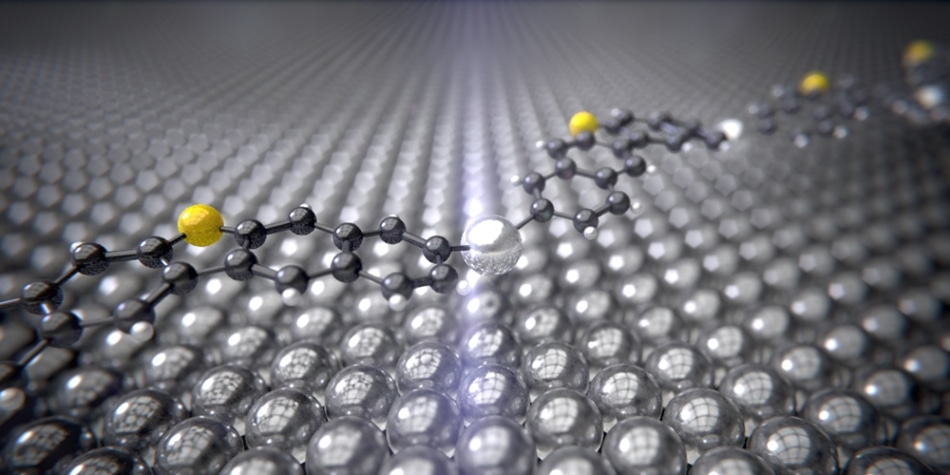 The intermediate product of the Ullmann reaction with the silver catalyst (silver) between the carbon rings (black) and sulfur atoms (yellow) curves like a bridge over the silver surface. (Credit: University of Basel, Department of Physics)
The intermediate product of the Ullmann reaction with the silver catalyst (silver) between the carbon rings (black) and sulfur atoms (yellow) curves like a bridge over the silver surface. (Credit: University of Basel, Department of Physics)
University of Basel physicists have successfully used an atomic force microscope to observe the working of a silver catalyst for the very first time.
The researchers have been able to evaluate the energy turnover and could possibly optimize the catalysis, based on the observations they made during an Ullmann reaction. The research, which was conducted by researchers from Iran and Japan, has been reported in the scientific journal Small.
The chemical reaction where the silver atoms catalyze the connection between two carbon atoms which were earlier bonded to iodine is called Ullmann reaction. Since 1901, researchers have been aware of and have exploited this type of reaction for numerous major chemical conversions, they were not able to examine the reaction’s intermediate product in detail before now.
The team, headed by Professor Ernst Meyer and Dr. Shigeki Kawai from the Swiss Nanoscience Institute and the Department of Physics at the University of Basel, used an atomic force microscope to successfully show the reaction at atomic resolution.
The observation revealed interesting information. The silver atoms react at a temperature of approximately -120oC with the molecules, seemingly curving like a bridge across a river. The next and final stage of the reaction involves the formation of the end product, where the silver atoms become free once again, resulting in the bonding of two carbon atoms.
Calculating Energy
For many years, scientists have used the Ullmann reaction for chemical reaction. Due to their use in binding organic molecules to various surfaces and creating solvent-free polymers, this reaction has once again caught the interest of researchers in the recent times.
Detailed examinations of the mechanisms of the catalysts’ work allow scientist to gain a better understanding of the reaction process.
Studies conducted in the past were not successful in displaying the special placement of the organometallic intermediate product. The comprehensive images obtained now provide an opportunity, for the first time, for study partner Professor Stefan Goedecker from the Department of Physics, University of Basel, to evaluate the energy turnover of the Ullmann reaction analyzed. The information collected reaffirms the intermediate product’s uncommon spatial arrangement and shows how the reaction can be tweaked.
Relatively Low Temperatures
The flexibility and curving of the molecule, observed during the experiment, is the probable reason for the comparative low temperature of 105oC, required by the reaction. The molecules undergo mechanical tension and as such react, at low temperatures, more easily.
If it is possible to make other catalysts produce such intermediate products, which are subject to tension, then catalyst reactions at lower temperatures can also be possible. As conventional catalysts such as rhodium, palladium or platinum mostly need high temperatures of 500oC to operate, leading to waste gases emission in a cold state, this measure is both economically and ecologically beneficial.
Various institutes such as the University of Tokyo (Japan), Shahid Beheshti University (Iran), the Japan Science and Technology Agency (Japan), the National Institute of Materials Science (Japan) and the Department of Physics at the University of Basel collaborated on this project.