Sep 14 2016
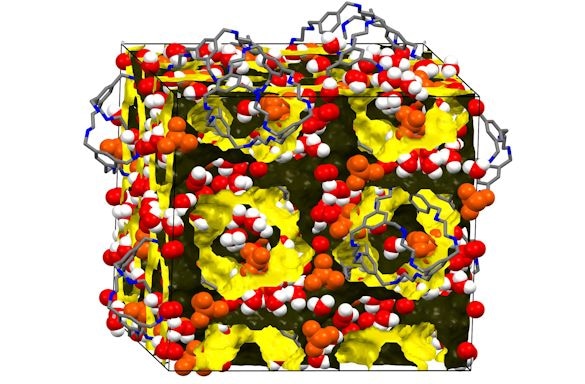 The structure of one of the cage crystals loaded with water. (Credit: University of Liverpool)
The structure of one of the cage crystals loaded with water. (Credit: University of Liverpool)
Scientists from the University of Liverpool have discovered a significant breakthrough that could lead to improved fuel cell materials being developed.
A research paper published by the scientists in Nature Communications explains how they synthesized nanometer-sized cage molecules capable of transferring charge in proton exchange membrane applications.
Proton-exchange membrane fuel cells (PEMFCs) are a potential technology for efficient and clean power generation in the twenty-first century.
A proton exchange membrane (PEM) present in PEMFCs is a component that carries positively-charged protons from the cell’s positive electrode to the negative one. Several PEMs are found to be hydrated and the charge is transmitted through networks of water inside the membrane.
An understanding about how the membrane’s structure enables protons to effortlessly move through it will result in improved PEM materials being designed. However, a number of PEMs are produced from amorphous polymers, making it difficult to analyze how protons are conducted as the exact structure is not known.
Researchers from the University’s Department of Chemistry synthesized molecules that surround an internal cavity, developing a porous organic cage where other smaller molecules can be loaded into, such as carbon dioxide or water. When the cages produce solid materials, they can align to form channels in which the small ‘guest’ molecules can pass through from one cage to another.
The material develops crystals where the alignment of cages is extremely regular. This enabled the researchers to construct a clear-cut description of the structure using crystallography, which allows the positions of atoms to be located. The molecules have also proven to be soluble in common solvents - they could be integrated with various other materials and fabricated into membranes.
The protonic conductivity of these porous organic cages was measured after the channels with water were loaded in order to test their viability as PEM materials. The cages displayed proton conductivities of up to 10-3 Scm, which is comparable to few of the best porous framework materials in the literature.
The scientists collaborated with researchers from the Defence Science and Technology Laboratory (DSTL), Center for Neutron Research at National Institute of Standards and Technology (NIST), and the University of Edinburgh and used a combination of experimental measurements and computer simulations to develop an enhanced picture how protons are conducted by the cage molecules.
Two unique characteristics of the proton conduction in organic cage crystals were treated as design principles for PEM materials in the years to come. All of the cages are initially positioned to allow the extension of the channels in three dimensions. This highlights that the movement of the protons is not restricted to a specific direction, as seen in the case of several porous materials tested until now.
Secondly, the cages monitor the movement of the water molecules, which highlights that protons are capable of traveling between them in a rapid manner. The cages seem to be flexible enough to permit reorganizing of the water, which is also significant when protons are transferred from one water molecule to the next molecule over longer distances.
In addition to introducing a new class of proton conductors, this study highlights design principles that might be extended to future materials. For example, the ‘soft confinement’ that we observe in these hydrated solids suggests new anhydrous proton conductors where a porous cage host positions and modulates the protonic conductivity of guest molecules other than water. This would facilitate the development of high temperature PEMFCs, as water loss would no longer be a consideration.
Dr Ming Liu, University of Liverpool
Liverpool Chemist, Dr Sam Chong, added: “The work also gives fundamental insight into proton diffusion, which is widely important in biology.”
Recently, Dr Chong was appointed as a lecturer in the University’s Materials Innovation Factory (MIF). Due to open in 2017, the £68M facility aims to revolutionize materials chemistry research and development by enabling the discovery of new materials, which are capable of saving natural resources and energy, enhancing health or altering a wide range of manufacturing processes
The paper `Three-dimensional Protonic Conductivity in Porous Organic Cage Solids’ is featured in Nature Communications.