Sep 21 2016
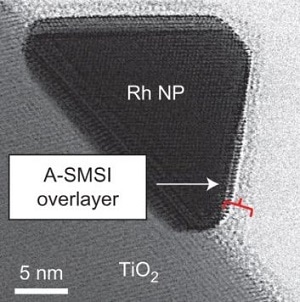 Atomic resolution electron microscopy showing the optimization of a carbon dioxide conversion reaction mediated by the catalyst (Rh nanoparticles, NP, on TiO2) and its unique surface interactions (A-SMSI) (Credit: UCR)
Atomic resolution electron microscopy showing the optimization of a carbon dioxide conversion reaction mediated by the catalyst (Rh nanoparticles, NP, on TiO2) and its unique surface interactions (A-SMSI) (Credit: UCR)
Industrial catalysts in the future will be able to control how chemical reactions work and determine the quantity of the product made, in addition to improving the reaction speed.
A group of scientists, headed by Philip Christopher, assistant professor of chemical and environmental engineering at the University of California, Riverside’s Bourns College of Engineering, demonstrated this and the working of these catalysts in a paper published in the September 19 issue of Nature Chemistry.
The paper titled “Adsorbate-mediated strong metal-support interactions in oxide-supported Rh catalysts,” describes the new method to dynamically control the operation of a catalyst. This allows the scientists to control and improve the product of the reaction.
The team included scientists from the University of Columbia, and University of California, Irvine. Advanced spectroscopy and microscopy approaches were used to demonstrate the work of the catalyst at an atomic scale.
The scientists executed a major chemical reaction that involves the conversion of carbon dioxide to synthetic natural gas and carbon monoxide. This reaction not only offers the potential to remove harmful atmospheric carbon dioxide, but the natural gas and carbon monoxide can be used as fuel and chemical precursors, respectively.
The researchers focused on gaining a better insight into how the catalyst drives the reaction at the atomic scale. This will enable scientists to change the properties of the catalysts to increase its efficiency in the reaction.
Christopher stated that the research can create new opportunities for carbon dioxide conversion chemistry, and that the visualization and dynamic tuning used in this study can be applied for other significant chemical processes.
The real uniqueness of the paper was being able to observe what was happening at an atomic scale and how physical changes in the catalyst affected the outcome of the carbon dioxide conversion reaction. The insights we gained pave the way for the design of more effective processes to produce fuels and chemicals.
Philip Christopher, Assistant Professor, University of California
The lead author of the paper is John Matsubu, who is a graduate student of chemical engineering in Christopher’s lab. Leo DeRita, also a graduate student in chemical engineering at UCR; Shuyi Zhang, George Graham and Xiaoqing Pan from the University of California Irvine; and Nebojsa Marinkovic and Jingguang Chen from Columbia University were also a part of the research. The National Science Foundation and the U.S. Department of Energy provided the financial support for the project.