Nov 17 2016
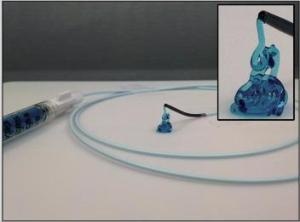 Image highlighting the injectability of the biomaterial through a catheter. The biomaterial can maintain its shape upon injection, only becoming liquid after a force is applied. Inset is a zoomed image of the STB extruded from the catheter tip. (Credit: BWH)
Image highlighting the injectability of the biomaterial through a catheter. The biomaterial can maintain its shape upon injection, only becoming liquid after a force is applied. Inset is a zoomed image of the STB extruded from the catheter tip. (Credit: BWH)
Clinicians often use small metallic coils to treat gastrointestinal bleeding, aneurysm or other such uncontrollable hemorrhaging. These coils can be inserted into a blood vessel permanently and prevent further bleeding. However, there are certain limitations to such coils.
Break-through bleeding can be experienced by patients who cannot form blood clots for some reason or if they are on blood thinning medicines, and rebleeding can occur in as many as 47% of the patients.
A team of bioengineers at Brigham and Women’s Hospital, headed by Ali Khademhosseini, PhD, worked with Rahmi Oklu MD, PhD, FSIR, a clinician who is an interventional radiologist (previously worded at Massachusetts General Hospital, currently working at Mayo Clinic).
The collaborative work led to the development of a quickly deployable hydrogel that can maintain its shape inside a blood vessel, preventing bleeding even in patients whose bodies cannot form blood clots. A paper published in the Science Translational Medicine on November 16 describes this newly developed agent.
This work is an example of how bioengineering can help address the challenges that clinicians and patients face. Our work thus far has been in the lab, but we are on a translational path to bring this new biomaterial for embolization to the clinic to improve patient care.
Ali Khademhosseini, PhD., Bringham and Women's Hospital
Known as the shear-thinning biomaterial (STB), the new agent is similar to toothpaste in consistency. It is made of nanoparticles and gelatin, which gives the material its gel-like properties. The biomaterial can flow into the blood vessel using a catheter but can hold its shape once within the vessel, blocking the aneurysm or vessel without depending on the clotting of blood.
The team performed mechanical tests in the lab and monitored the changes of the STB to optimize its properties in animal models. The STB was then tested in rodent and porcine models; the blood vessels of the latter have similar dimensions as that of human blood vessels.
Its ability to remain at the place of injection, to withstand pressure inside the blood vessel, and naturally degrade over a period of time are some of the advantageous properties of STB. The team also found that the cells were attracted by the material to move and deposit themselves at the place of the STB, helping in blocking the vessel.
The individual component materials that are used to make the STB have previously been used in humans; this makes their regulatory processes and clinical use much easier.
The team next intends to start clinical trial to test the efficiency and safety of the STB for use in humans.
The U.S. Army Research Office, Sloan Foundation, Mayo Clinic the National Institutes of Health and National Science Foundation have supported this research.