Jan 27 2017
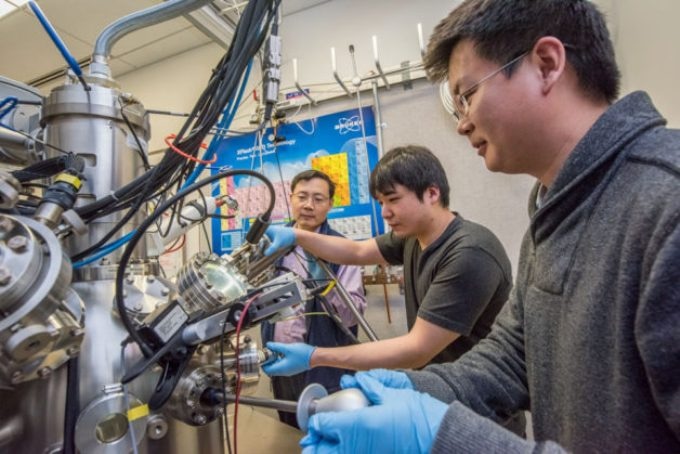 Berkeley Lab scientists Junqiao Wu, Changhyun Ko, and Fan Yang (l-r) are working at the nano-Auger electron spectroscopy instrument at the Molecular Foundry, a DOE Office of Science User Facility. They used the instrument to determine the amount of tungsten in the tungsten-vanadium dioxide (WVO2) nanobeams. CREDIT: Marilyn Chung/Berkeley Lab.
Berkeley Lab scientists Junqiao Wu, Changhyun Ko, and Fan Yang (l-r) are working at the nano-Auger electron spectroscopy instrument at the Molecular Foundry, a DOE Office of Science User Facility. They used the instrument to determine the amount of tungsten in the tungsten-vanadium dioxide (WVO2) nanobeams. CREDIT: Marilyn Chung/Berkeley Lab.
Among materials, there is a familiar anomaly, a nonconforming metal, the properties of which have been discovered through a new study carried out by an international research team including scientists from the Department of Energy’s (DOE) Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California, Berkeley.
The researchers have found that electrons in vanadium dioxide have the ability to conduct electricity without conducting heat.
The outcomes are published in the Science journal, and can open the door to a broad array of applications such as thermoelectric systems in which waste heat from appliances and engines is converted into electricity.
The Wiedemann-Franz Law governs the relationship between thermal and electrical conductivity in most of the metals. According to the law, good conductors of electricity are good conductors of heat as well. However, this is not the case for metallic vanadium dioxide, which has a peculiar property of transforming from an insulator to a metal at a temperature of 67 °C (152 °F).
This was a totally unexpected finding. It shows a drastic breakdown of a textbook law that has been known to be robust for conventional conductors. This discovery is of fundamental importance for understanding the basic electronic behavior of novel conductors.
Junqiao Wu, Physicist, Berkeley Lab
Wu and his colleagues worked with Olivier Delaire from DOE’s Oak Ridge National Laboratory and an associate professor at Duke University, to analyze the characteristics of vanadium dioxide. The research team were able to discover the proportion of thermal conductivity that was the result of not only vibration of the crystal lattice, called phonons, of the material but also the movement of electrons using the outcomes of X-ray scattering experiments and simulations.
Astonishingly, they discovered that the thermal conductivity due to the electrons was 10 times smaller than that explained by the Wiedemann-Franz Law.
The electrons were moving in unison with each other, much like a fluid, instead of as individual particles like in normal metals. For electrons, heat is a random motion. Normal metals transport heat efficiently because there are so many different possible microscopic configurations that the individual electrons can jump between. In contrast, the coordinated, marching-band-like motion of electrons in vanadium dioxide is detrimental to heat transfer as there are fewer configurations available for the electrons to hop randomly between.
Junqiao Wu, Physicist, Berkeley Lab
Notably, the amount of heat and electricity that can be conducted by vanadium dioxide can be adjusted by mixing other materials to it. Upon doping single crystal vanadium dioxide samples with tungsten metal, the researchers were able to reduce the phase transition temperature at which vanadium dioxide transforms into a metal.
At the same time, the electrons in the metallic phase became better heat conductors. This meant that the researchers could control the amount of heat that can be dissipated by vanadium dioxide by switching, at tunable temperatures, the material’s phase from insulator to metal and vice versa.
The researchers said that such materials can be used to dissipate or scavenge the heat generated in engines, or they can also be formed into a window coating that enables efficient utilization of energy in buildings.
This material could be used to help stabilize temperature. By tuning its thermal conductivity, the material can efficiently and automatically dissipate heat in the hot summer because it will have high thermal conductivity, but prevent heat loss in the cold winter because of its low thermal conductivity at lower temperatures.
Fan Yang, co-lead author of the study, who is a postdoctoral researcher at Berkeley Lab’s Molecular Foundry, a DOE Office of Science User Facility at which a portion of the research was carried out.
Another advantage of vanadium dioxide is that it occurs in a transparent state below the temperature of about 30 °C, or 86 °F, and has the ability to absorb infrared light above the temperature of 60 °C, or 140 °C.
According to Yang, further analysis has to be made before the commercialization of vanadium dioxide, adding that the research underscores the ability of a material with “exotic electrical and thermal properties.”
Although there are a few other materials (apart from vanadium dioxide) with the ability to conduct electricity better than heat, those materials exist at temperatures that are hundreds of degrees below zero. The researchers stated that this renders it highly difficult to develop these materials into real-time applications.
Sangwook Lee from Kyungpook National University in South Korea, Kedar Hippalgaonkar from the Institute of Materials Research and Engineering in Singapore, and Jiawang Hong from the Beijing Institute of Technology in China are the other co-lead authors of the study. Lee and Hippalgaonkar began working on the study while they were postdoctoral researchers at UC Berkeley. Hong started working when he was a postdoctoral researcher at Oak Ridge National Laboratory.
Facilities supported by the Electronic Materials Program at DOE’s Office of Science additionally supported the research.