Jun 8 2017
For the first time, Columbia Engineering Scientists have developed an innovative method that is based on the nacre of oyster shells, which is a composite material with exceptional mechanical properties such as high resilience and strength.
The Research team was successful in manipulating the way in which the nanoparticles get self-assembled into structures at three highly disparate length scale regimes. They achieved this by altering the crystallization speed of a polymer thoroughly combined with nanoparticles at the start. The multiscale ordering rendered the base material stiffer by nearly an order of magnitude while retaining the desired lightweight behavior and deformability of the polymeric materials. The research was headed by Sanat Kumar, Bykhovsky Professor of Chemical Engineering, and has been published online in ACS Central Science on 7th June 2017.
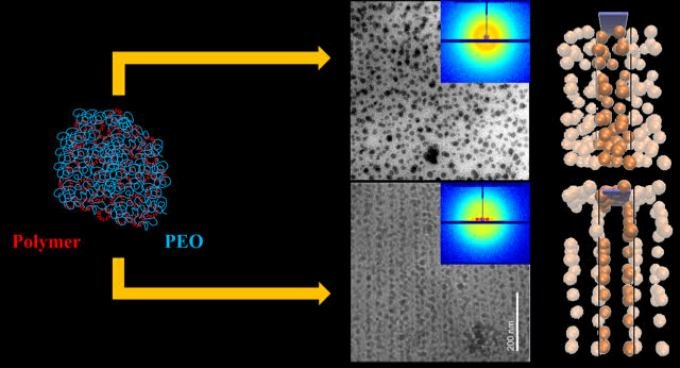 This figure illustrates that polymer crystallization speed can be used to control the spatial distribution of nanoparticles. Impurities (here, the nanoparticles) will become engulfed by the crystal if it grows too rapidly. However, when the rate slows, the crystal will expel the defects. CREDIT: Sanat Kumar.
This figure illustrates that polymer crystallization speed can be used to control the spatial distribution of nanoparticles. Impurities (here, the nanoparticles) will become engulfed by the crystal if it grows too rapidly. However, when the rate slows, the crystal will expel the defects. CREDIT: Sanat Kumar.
Essentially, we have created a one-step method to build a composite material that is significantly stronger than its host material. Our technique may improve the mechanical and potentially other physical properties of commercially relevant plastic materials, with applications in automobiles, protective coatings, and food/beverage packaging, things we use every day. And, looking further ahead, we may also be able to produce interesting electronic or optical properties of the nanocomposite materials, potentially enabling the fabrication of new materials and functional devices that can be used in structural applications such as buildings, but with the ability to monitor their health in situ.
Professor Sanat Kumar, Bykhovsky Professor of Chemical Engineering, Columbia University
Nearly 75% of commercially used polymers ‒ such polypropylene used for making bottles and polyethylene for packaging ‒ are semicrystalline. The low mechanical strength of these materials render them unfit to be applied for various advanced applications, including automobile fittings such as fanbelts, tires, bumpers and so on. From the early 1990s, Scientists had knowledge of the fact that altering the nanoparticle dispersion in metal, polymer and ceramic matrices can drastically enhance material characteristics. An excellent natural precedent to this is nacre, made of 5% crystalline polymer (chitin) and 95% inorganic aragonite; its mechanical properties are highly enhanced by its hierarchical nanoparticle ordering, that is, a mixture of thin layers of elastic biopolymers and intercalated brittle platelets. Moreover, a nanoscale, that is, ~10 nm thick, crystalline biopolymer layer holds the parallel aragonite layers together to form “bricks” that eventually get assembled into “brick-and-mortar” superstructures at the micrometer and larger scales. With numerous length sizes, the structure largely enhances the toughness of the material.
While achieving the spontaneous assembly of nanoparticles into a hierarchy of scales in a polymer host has been a ‘holy grail’ in nanoscience, until now there has been no established method to achieve this goal. We addressed this challenge through the controlled, multiscale assembly of nanoparticles by leveraging the kinetics of polymer crystallization.
Dan Zhao, First Author of the Paper and Kumar’s PhD student
Although Scientists working on polymer nanocomposites have achieved facile control of nanoparticle organization in an amorphous polymer matrix, that is, the polymer does not crystallize, until now none of them have been successful in tuning the assembly of nanoparticles in a crystalline polymer matrix. One related approach relied on ice-templating, through which investigators have been able to crystallize smaller molecules, mainly water, to assemble colloid particles. However, the intrinsic kinetics of such processes render the particles to be usually pushed out into the microscale grain boundaries, hence Scientists have been unable to assemble the nanoparticles over the multiple scales required to resemble nacre.
Researchers in Kumar’s team are highly skilled in tuning the structure and hence the characteristics of polymer nanocomposites and they discovered that when nanoparticles are mixed in a polymer (i.e. polyethylene oxide) solution and the crystallization speed is altered by modifying the sub-cooling degree, that is, how much lower than the melting point the crystallization occurred, the way in which the nanoparticles get self-assembled into three different scale regimes ‒ macro, micro, nano-meter ‒ could be controlled. Each of the nanoparticles were evenly surrounded by the polymers and evenly spaced before the crystallization was initiated. Subsequently, the nanoparticles assembled into 10-100 nm sheets and the sheets arranged into aggregates on the microscale, that is, 1-10 mm, upon crystallization of the polymer.
This controlled self-assembly is important because it improves the stiffness of the materials while keeping them tough. And the materials retain the low density of the pure semicrystalline polymer so that we can keep the weight of a structural component low, a property that is critical to applications such as cars and planes, where weight is a critical consideration. With our versatile approach, we can vary either the particle or the polymer to achieve some specific material behavior or device performance.
Professor Sanat Kumar, Bykhovsky Professor of Chemical Engineering, Columbia University
Subsequently, Kumar and his colleagues intend to investigate the basic principles that allow particles to transcend toward specific regions of the system, and to devise techniques to speed up the kinetics of particle ordering, which at present takes a few days. Then, the Researchers intend to analyze other application-driven particle/polymer systems, for example, nanoparticle/polylactide systems that can be designed to be next-generation sustainable and biodegradable polymer nanocomposites, or polyethylene/silica applied in buildings, car bumpers and bridges.
“The potential of replacing structural materials with these new composites could have a profound effect on sustainable materials as well as our nation’s infrastructure,” stated Kumar.