Jun 27 2017
Soon high-definition displays will function with a new type of semiconductor. Researchers at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) have demonstrated that a class of semiconductor known as halide perovskites is capable of discharging multiple, bright colors from just one nanowire at resolutions as small as 500 nm.
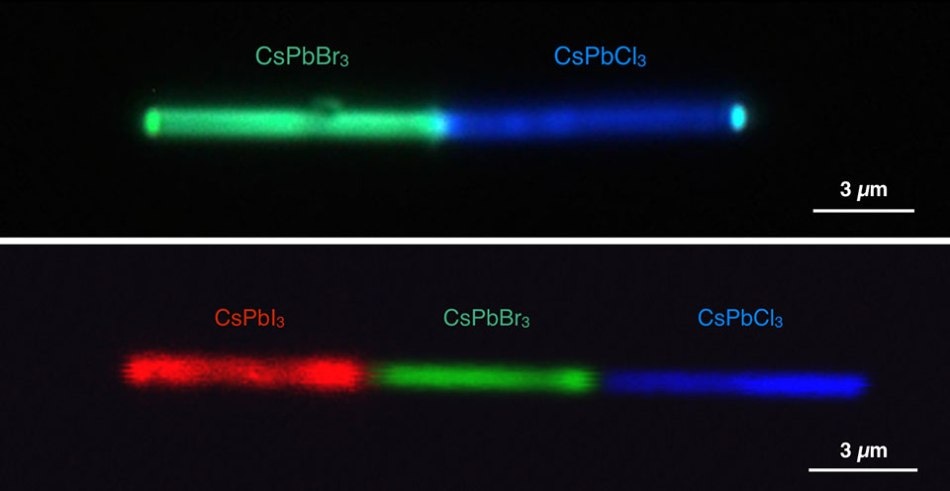 Single nanowires shown emitting different colors. The top panel shows a cesium lead bromide (CsPbBr3)-cesium lead chloride (CsPbCl3) heterojunction simultaneously emitting green and blue lights, respectively, under UV excitation. The bottom panel shows a cesium lead iodide (CsPbI3)-cesium lead bromide-cesium lead chloride configuration emitting red, green, and blue lights, respectively. (Credit: Letian Dou/Berkeley Lab and Connor G. Bischak/UC Berkeley)
Single nanowires shown emitting different colors. The top panel shows a cesium lead bromide (CsPbBr3)-cesium lead chloride (CsPbCl3) heterojunction simultaneously emitting green and blue lights, respectively, under UV excitation. The bottom panel shows a cesium lead iodide (CsPbI3)-cesium lead bromide-cesium lead chloride configuration emitting red, green, and blue lights, respectively. (Credit: Letian Dou/Berkeley Lab and Connor G. Bischak/UC Berkeley)
The research findings have been published online this week in the early edition of the Proceedings of the National Academy of Sciences. The research signifies a clear challenge to quantum dot displays that depend upon traditional semiconductor nanocrystals to discharge light. It could also impact the development of new applications in optoelectronics, nanoscopic lasers, photovoltaics and ultrasensitive photodetectors, among others.
Electron beam lithography was used by the Researchers to fabricate halide perovskite nanowire heterojunctions, the junction of two types of semiconductors. In device applications, heterojunctions establish energy level and bandgap features, and are thus thought to be a chief building block of current photovoltaics and electronics.
The Researchers highlighted that the lattice in halide perovskites is held together by ionic instead of covalent bonds. Atoms of opposite charges are attracted and transfer electrons to each other in ionic bonds. In contrast, covalent bonds occur when atoms share their electrons with each other.
With inorganic halide perovskites, we can easily swap the anions in the ionic bonds while maintaining the single crystalline nature of the materials. This allows us to easily reconfigure the structure and composition of the material. That’s why halide perovskites are considered soft lattice semiconductors. Covalent bonds, in contrast, are relatively robust and require more energy to change. Our study basically showed that we can pretty much change the composition of any segment of this soft semiconductor.
Peidong Yang, Senior Faculty Scientist at Berkeley Lab’s Materials Sciences Division and Chief investigator of the study
Here, the team analyzed cesium lead halide perovskite, and then they used a common nanofabrication method integrated with anion exchange chemistry to switch out the halide ions to form cesium lead bromide, cesium lead iodide and cesium lead chloride perovskites.
Each difference resulted in a different color discharged. Furthermore, the Researchers demonstrated that a number of heterojunctions could be engineered on a single nanowire. They were able to achieve a pixel size down to 500 nm, and they established that the color of the material was tunable all the way through the entire range of visible light.
The Researchers said that the chemical solution-processing method used to treat this class of soft, ionic-bonded semiconductors is a lot simpler than approaches used to make traditional colloidal semiconductors.
For conventional semiconductors, fabricating the junction is quite complicated and expensive. High temperatures and vacuum conditions are usually involved to control the materials’ growth and doping. Precisely controlling the materials composition and property is also challenging because conventional semiconductors are ‘hard’ due to strong covalent bonding.
Letian Dou, the Study’s Co-lead Author
To switch the anions in a soft semiconductor, the material is put into a special chemical solution at room temperature.
“It’s a simple process, and it is very easy to scale up,” said Yang, who is also a Professor of Chemistry at UC Berkeley. “You don’t need to spend long hours in a clean room, and you don’t need high temperatures.”
The Researchers are continuing to enhance the resolution of these soft semiconductors and are keen on incorporating them into an electric circuit.
Other Co-lead Authors on this paper are Christopher Kley, UC Berkeley Postdoctoral Fellow, and Minliang Lai, UC Berkeley Graduate Student. Dou is now an Assistant Professor of Chemical Engineering at Purdue University.
This study was supported by the DOE Office of Science.