Jul 20 2017
Scientists from EPFL have developed a uranium-based complex that can allow reactions of nitrogen fixation to occur in ambient conditions. This work overcomes one of the major challenges in building more efficient industrial-scale nitrogen products similar to ammonia.
 This is an illustration of how the uranium-based compound developed in this study would work. CREDIT: Marinella Mazzanti/EPFL
This is an illustration of how the uranium-based compound developed in this study would work. CREDIT: Marinella Mazzanti/EPFL
Nitrogen, which is naturally abundant, is the fundamental constituent of various beneficial products, including artificial as well as natural products. For this to occur, a reaction called “nitrogen fixation” in which molecular nitrogen is disintegrated into two nitrogen atoms that can be subsequently coupled with other elements such as hydrogen or carbon is necessary.
However, carrying out nitrogen fixation to industrially produce ammonia involves drastic conditions under extremely high pressure and temperature.
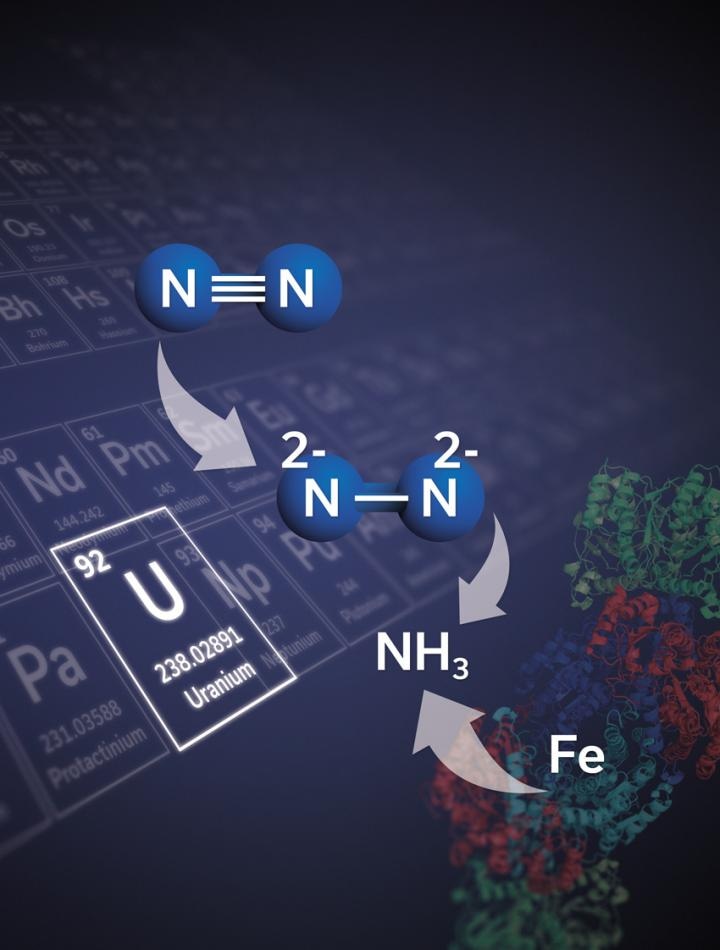
At present, Researchers at EPFL have designed a uranium-based compound that enables nitrogen fixation to be carried out in ambient atmosphere. The study was reported in the journal Nature and can prove to be the foundation through which highly efficient catalysts can be synthesized. The study also emphasizes innovative ideas that can be applied to metals other than uranium.
Although ammonia is extensively used, it cannot be synthesized easily. The prevalent and principal technique for industrial production of ammonia is the Haber-Bosch process. The process involves using an iron-based catalyst at a pressure of around 300 bar, which is nearly 300 times the sea level pressure and a temperature of about 450 °C.
This is because molecular nitrogen contained in ambient air does not easily react with other elements, thereby making nitrogen fixation greatly difficult. At the same time, a number of microorganisms have the ability to carry out nitrogen fixation under regular conditions, within the delicate walls of a cell. They perform this by means of enzymes which have biochemically intrigued Chemists for usage in industrial applications.
A complex including two uranium(III) ions as well as three potassium centers, connected by a flexible metalloligand framework and a nitride group, was produced in Marinella Mazzanti’s lab at EPFL.
Such a system has the ability to bind nitrogen and disintegrate into nitrogen atoms under normal, ambient conditions when hydrogen and/or protons/carbon monoxide is added to the ensuing nitrogen complex. Consequently, the molecular nitrogen is split and gets naturally bonded with carbon and hydrogen.
The research has demonstrated that a molecular uranium complex has the ability to convert molecular nitrogen into value-added compounds even without the drastic conditions mandated in the Haber-Bosch process.
Moreover, the study paves the way for producing nitrogen compounds other than ammonia, and acts as the starting point for formulating catalytic processes for synthesizing nitrogen-containing organic molecules from molecular nitrogen.