Jul 26 2017
A research team has conceived an easier and quicker technique to synthesize sulfur-containing polymers. The technique is believed to decrease the cost of large-scale synthesis.
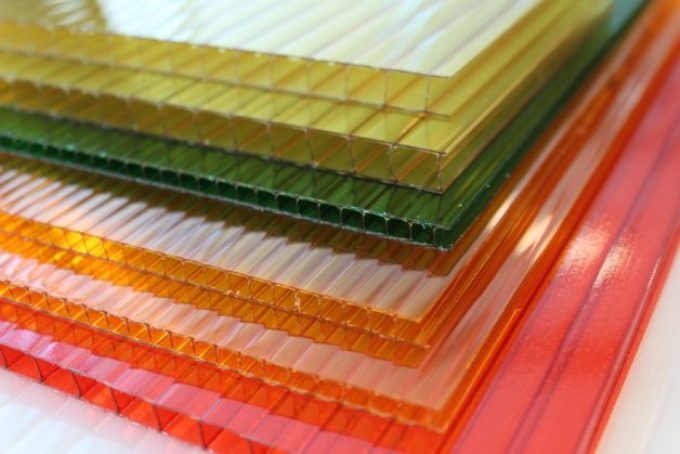 Researchers have developed a chemical process that could make a class of polymers known as polysulfates more competitive with polycarbonates—sturdy plastics that can form structural panels like the ones pictured here. Such panels can be used as building materials. CREDIT: falconsoft/Pixabay.
Researchers have developed a chemical process that could make a class of polymers known as polysulfates more competitive with polycarbonates—sturdy plastics that can form structural panels like the ones pictured here. Such panels can be used as building materials. CREDIT: falconsoft/Pixabay.
The outcomes of the research have been reported in the journals Angewandte Chemie and Nature Chemistry. The study paves the way for developing innovative products from this type of polymers and reducing the amount of hazardous waste byproducts. The reaction method devised by the research team (known as sulfur(VI) fluoride exchange, or SuFEx), integrated with a newly recognized type of catalysts that accelerate the reactions, can be applied to produce a range of products such as, mobile phone cases, medical devices, water bottles and bulletproof glass.
When a useful molecule is found, Chemists can apply some reactions that are efficacious and simple to satisfy the industrial production demands for achieving cost-efficient increase in production capacity. In the year 2001, K. Barry Sharpless, a Nobel laureate, launched an innovative idea in the field of organic chemistry, namely, “click chemistry.” The concept outlines a series of highly reactive, controllable reactions that ensure high yield and either decrease the need for or avoid the need for purification.
Similar to natural reactions, click reactions obey simple protocols, use easily accessible raw materials and function under gentle reaction conditions with gentle starting reagents. Click chemistry has turned out to be a beneficial tool for producing a broad range of prospectively profitable compounds as factories constantly work to find out innovative materials and drugs.
Researchers from Lawrence Berkeley National Laboratory’s (Berkeley Lab’s) Molecular Foundry—an establishment specifically concentrating on nanoscale science—collaborated with a research team headed by Sharpless and Peng Wu, Professors at the Scripps Research Institute (TSRI). The group employed a SuFEx click reaction to develop long chains of linked sulfur-containing molecules called polysulfonates and polysulfates.
Click chemistry is a powerful tool for materials discovery, but synthetic chemists are often not well-equipped to characterize the polymers they create. We can provide a broad spectrum of expertise and instrumentation that can expand the scope and impact of their research.
Yi Liu, Director of the Organic Synthesis Facility, the Molecular Foundry
The SuFEx reaction is a new class of click reactions introduced in the year 2014. It quickly and definitively produces new chemical bonds that connect compounds together with sulfonates or sulfates. Although polysulfates have been demonstrated to give stiff competition to polycarbonates—which are high-strength plastics used for making water bottles, eyewear lenses and so on — they have not been applied much for industrial usage as easily scalable and dependable synthetic processes are deficient.
In order to get the better of mass-production challenges of polysulfonates and polysulfates, the TSRI Researchers investigated a range of starting reagents and catalysts to improve the SuFEx reaction. For analyzing physical characteristics and to ascertain whether the newly developed polymers are thermally stable, the Researchers sought the assistance of their colleagues from the Molecular Foundry.
Similar to forming a necklace by repeating a specific pattern of beads, polymers are synthesized from smaller molecules. For synthesizing a polysulfonate “necklace” by means of SuFEx reaction, the research recognized bisphenol ether and ethenesulfonyl fluoride-amine/aniline to be the perfect “beads” to use and discovered that when bifluoride salt was used as the catalyst made, the earlier slow reaction “click” was put into action. The team also discovered that the higher efficaciousness of the reaction culminated in an astonishing 99% conversion of starting reactants into products within just an hour.
They also noted that the new reaction reduced the amount of catalyst required by 100 to 1000 times than other familiar techniques, thus leading to considerable reduction in the hazardous waste products. Moreover, bifluoride salts are considerably less corrosive when compared to catalysts used earlier, enabling the use of a broad array of starting reactant “beads”—the team believed that these can be used for various industrial processes.
There are many new polymers that haven’t been widely used by industry before. By reducing waste and improving product purity, we lower the cost and make this reaction much more industry friendly.
Yi Liu, Director of the Organic Synthesis Facility, the Molecular Foundry
The Molecular Foundry is a DOE Office of Science User Facility that offers free access to ultra-modern equipment and multidisciplinary proficiency in the field of nanoscale science to visiting Researchers from across the globe.
The National Science Foundation, the National Institutes of Health, the Chinese Natural Science Foundation and the Jiangsu Province Office of Education in China supported the research.