Customers in world-renowned research, QA/QC, and educational laboratories have relied on the exceptional value and high-performance delivered by Thermo Scientific™ UV-Visible spectrophotometers for more than 60 years.
The Thermo Scientific™ Evolution™ 201 and 220 UV-Visible Spectrophotometers enable simplified workflows; a variety of accessories and cutting-edge technology to produce the high-quality results you depend on, consistently.
The Thermo Scientific™ Evolution™ 260 Bio UV-Visible Spectrophotometer adds techniques for quantifying nucleic acids and proteins and carrying out thermal denaturation studies if pre-programmed Life Science techniques are required.
The Thermo Scientific™ Evolution™ 350 UV-Visible Spectrophotometer provides solutions that are designed to simplify the sophisticated for your advanced applications if additional operational flexibility and advanced performance are required.

Evolution UV-Visible Pharmacopeia Performance Requirements
For UV-Visible spectrophotometers in regulated laboratories, performance verification at installation and at set intervals thereafter is needed. National pharmacopeias with international reach define specific performance levels for a number of criteria in the pharmaceutical industry.
Due to its superior specifications, Evolution 350 will meet the performance needs for all global pharmacopeias including Japanese Pharmacopoeia (JP), European Pharmacopoeia (EP), and the United States Pharmacopoeia (USP).
The Evolution 200 series will now meet all requirements for EP and USP because of changes in manufacturing to improve the specifications.
Table 1.
| Instrument |
USP |
EP |
JP |
| Evolution 201 |
✓ |
✓ |
|
| Evolution 220 |
✓ |
✓ |
|
| Evolution 260 Bio |
✓ |
✓ |
|
| Evolution 350 |
✓ |
✓ |
✓ |
Pharmacopeia Performance Verification Testing
Thermo Scientific has the appropriate standards options for any pharmacopeia need, no matter the operational range. The new USP and EP UV Standards Set includes the full list of standards that are required for performance verification for qualification in the UV region.
Add the new USP and EP Visible Standards Set if assessing the performance of the instrument in the visible region is required.
Table 2.
| |
222-327200 USP and EP
UV Standards Set |
222-327300 USP and EP
Visible Standards Set |
| Operating Range |
<400 nm |
≥400 nm |
| Wavelength Accuracy and Precision |
Holmium Oxide Solution |
Didymium Glass |
Photometric Accuracy,
Precision and Linearity |
Three Potassium Dichromate
Standards |
Four Neutral Density Glass
Standards |
| Resolution |
Toluene in Hexane Solution |
N/A |
| Stray Light |
Potassium Chloride Solution
Sodium Iodide Solution
Sodium Nitrate Solution |
N/A |
The Qualification Filter Kit for UV-Visible can be utilized to fully qualify a spectrophotometer if only general instrument qualification is required instead of full pharmacopeia performance verification.

The Qualification Filter Kit for UV-Visible possesses some of the standards employed for USP and EP performance verification, to supplement this kit, additional standards can be purchased at a later date.

An optional mercury lamp accessory is available for purchase for each class of instrument.
- Selecting the mercury lamp accessory frees customers who carry out routine performance verification testsManchester2020!
- from the burden or purchasing and re-certifying standard solutions for measuring these parameters. A mercury lamp never needs calibration, as a fundamental physical standard.
- For determining wavelength accuracy, both European and US Pharmacopoeias call out low-pressure mercury emission lamps as the preferred choice.
- A pre-installed mercury lamp option is available for the Evolution 350. Stand-alone mercury lamp accessories are also available for the Evolution 200 and Evolution 350 series.
Complete IQ/OQ Validation Package
Thermo Scientific Validator packages supply support for validation activities and system qualifications for software, instruments, and accessories.
For your convenience, all of the reference materials and documentation required to facilitate the compliance of a Thermo Scientific spectrophotometer system with the requirements of FDA, GxP, ISO 9001:2008 and ISPE 2001 guidelines and regulations are included.
For simple and efficient utilization, validator packages streamline Operational Qualification (OQ), Installation Qualification (IQ), and assist in Performance Qualification (PQ) procedures and development.

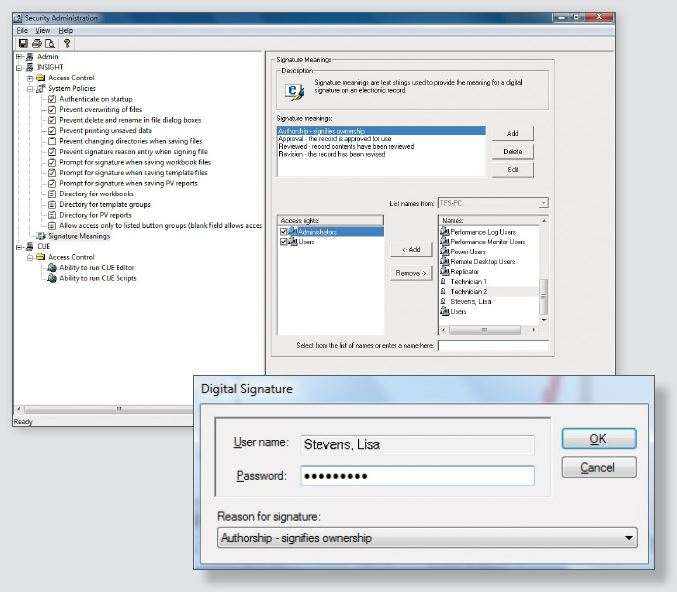
Reliable Data Security for Your Electronic Records
Insight Security software is here to simplify the process should your laboratory require 21 CFR Part 11 compliance. Insight Security software combines security and data integrity assurance with the versatility needed for your multi-user laboratory, utilizing the same easy-to-use interface.
- Even when the software is not running, ensure that any modifications to files associated with Insight Security are monitored and logged, with software that is seamlessly integrated with Microsoft® Windows® security features
- Quickly and easily manage your users’ privileges and access from one central server location with the cross-platform Thermo Scientific Security Suite software
- Attain clear traceability with sample history and electronic signature details which are stored directly within your protected data files
Integrated Informatics Solutions
Facilitating end-to-end traceability of samples and associated laboratory processes, supplying a central repository for data and test results, and ensuring regulatory compliance, laboratory informatics is a vital part of any laboratory.
Thermo Scientific Lab Information Management Systems (LIMS) supply a complete informatics solution for technique execution, laboratory, and data management.
Combined with their Integration Manager (IM), Laboratory Execution System (LES), and Scientific Data Management System (SDMS) Thermo Scientific LIMS provides the most comprehensive paperless lab solution available today.
Now lab managers and scientists across all industries can:
- Manage methods and workflow
- Achieve full instrument integration
- Retrieve and archive any kind of raw scientific data
- Export results across the organization
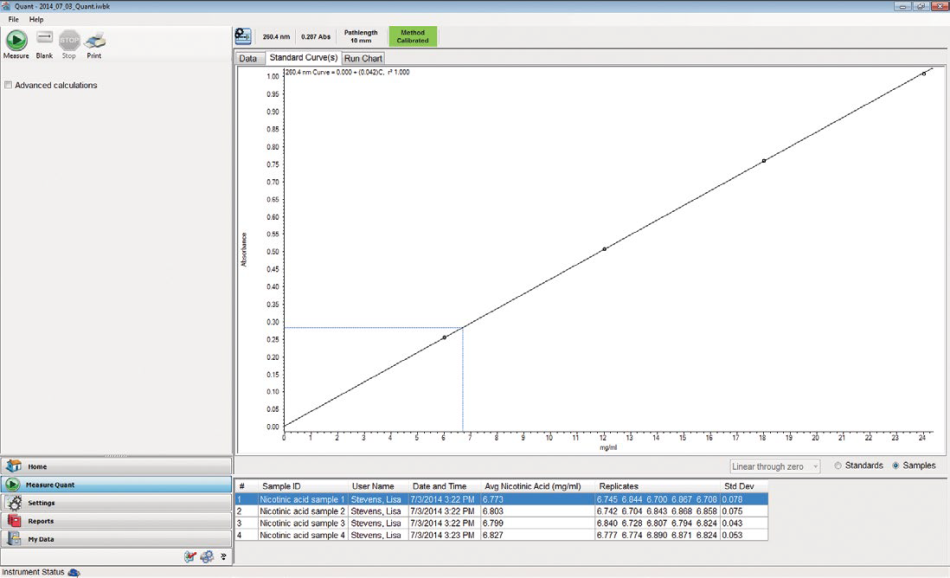
Reliable Precision for Quantitative Analysis
A key part of quality control analyses is reliable results. From standard curves based on peak area to simple, single-standard comparisons, Thermo Scientific has the tools to gather the answers required, every time.
Evolution UV-Visible spectrophotometer’s double beam optical design supplies precision performance, outstanding reliability, and minimal downtime.
- With double-beam optics and a reference position detector for monitoring control samples, you can enjoy long-term stability during data acquisition.
- Achieve more each day. The instant-on xenon flash lamp is guaranteed for three years of continuous use and requires no warm-up time.
Complete Pharma Solution Bundles Available
With numerous bundles available, Thermo Scientific possesses the ideal configuration to meet every requirement. Each bundle will include:
- Choice of USP and EP Standards Set or general Qualification Filter Kit
- Validator 2 IQ/OQ Qualification Documentation
- Choice of Evolution UV-Visible spectrophotometer
- INSIGHT with Security software
Pharma UV-Visible Instrumentation Checklist
- LIMS compatibility
- Meets pharmacopeia performance requirements
- 21CFR Part 11 for data integrity
- Advanced QA/QC software functionality
- IQ/OQ qualification package
- Validated installation

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific – Materials & Structural Analysis.
For more information on this source, please visit Thermo Fisher Scientific – Materials & Structural Analysis.