Rice is a dietary staple for more than half of the world’s population. Due to its high mineral content and gluten-free properties, the pulverized form is usually one of the first solid foods to be introduced to babies.
It is also the most commonly consumed grain for people with Celiac disease and for those following gluten-free diets.
In recent years, there has been some concern over the presence of high levels of arsenic (As) in rice, where this carcinogenic metalloid can accumulate at levels far exceeding ambient concentrations.1
There are several possible sources of As in rice, including the water used for irrigation, the soil in which it is grown, as well as any atmospheric fallout.
High concentrations of arsenic in water and soils can be a result of the natural geology of the area leaching this metalloid into the environment, or it can be introduced via a range of different anthropogenic activities.2
Inorganic As species (As III and As V) are the commonly found forms of As in the terrestrial environment and they are also the most toxic.
The reducing and anoxic conditions often seen in rice paddy fields can advance the interconversion and reduction of some As species to the most frequently used toxic and carcinogenic form of As (As III).
Once As from the surrounding environment is absorbed by the rice plant, it may be metabolized and methylated somewhat into a less toxic As species, where the extent of such methylation is reliant primarily upon a specific part of the plant (roots, shoots, leaves, seeds).
Accordingly, if evaluation of the overall concentration of As in rice crops were to be conducted, this would simply offer a sum of all of the various species of arsenic in the rice and may lead to inaccurate conclusions about the potential toxicity. It is for this reason that studies for speciation are necessary.
Since China produces the largest amount of rice in the world, in 2014 the Chinese government published a national standard method for food safety, GB 5009.11 to promote the evaluation of As species in rice and rice products.
In this paper, HPLC-ICP-MS is identified as the analytical technique of choice.
In 2017, GB 2762 was published, forcing maximum allowable limits (0.2 mg/kg) on the inorganic arsenic content. One of the challenges experienced in a number of As speciation methods is the need for the solvent gradient to have the ability to control the elution of the different arsenic species.
This necessitates a powerful plasma with precise impedance matching, which has the capacity to cope with the fluctuating solvent gradient and the influence this may have upon ionization.
Furthermore, since this method may accelerate the wear and tear on the pump seal, a pump with a mechanism that has the capacity to wash behind the pump seal would significantly reduce seal damage, reducing downtime and the necessity for maintenance visits.
Throughout this study, five arsenic species were determined in a commercial rice sample and certified reference material utilizing a gradient anion-exchange method in line with GB 5009.11. The analysis was conducted on a PerkinElmer NexSAR™ HPLC-ICP-MS speciation solution, incorporating a NexSAR inert HPLC paired with a NexION® ICP-MS.
Experimental
Sample Preparation
Preparation of calibration standards with concentrations of 0.2, 1, 5, 10, 50 and 100 µg/L was conducted in 0.15 M HNO3 (Ultrapure, 68%, Suzhou JINGRUI Chemical Co. Ltd., Suzhou, Jiangsu, China) using the following reagents:
- As III from arsenite (75.5±1.2 µg/g)
- As V from arsenate (17.5±0.4 µg/g)
- Monomethylarsonic acid (MMA; 25.1±0.8 µg/g)
- Dimethylarsinic acid (DMA; 52.9±1.8 µg/g)
- Arsenobetaine (AsB; 38.8±1.1 µg/g)
These all derived from the National Institute of Metrology, China (NIM, Beijing, China).
These particular species were chosen for the assessment due to the requirements of the national standard and their abundant presence in rice. Concentrations of the calibration standards were selected in line with the recommended concentrations as detailed in GB 5009.11.
Each species was determined according to its elution time by separately analyzing each arsenic species.
A commercial rice sample was bought from a local store and prepared for analysis. A certified reference material (CRM, GBW(E)100348, Beijing China) was used to validate the method.
The sample (0.5 g) was weighed precisely in triplicate on an analytical balance (Mettler Toledo, Columbus, Ohio, USA) to account for heterogeneity in the sample and set in a 15 mL polypropylene tube (Crystalgen, Commack, New York, USA).
Nitric acid (10 mL of 0.15 M HNO3) was introduced to each sample and the tube swirled to make sure that the sample was completely immersed in the acid. Subsequently, the samples were inserted into a drying oven (DHG-9000, Shanghai Bluepard Instruments Co. LTD. Shanghai, China) for three hours at 90 °C and manually shaken for one minute every half hour.
Post-extraction, the samples were left to cool and were centrifuged (TDL-60B, ShangHai Anting Scientific Instrument Factory, Shanghai, China) for 15 minutes at 8000 rpm.
The supernatant was poured and passed through a 0.45 µm syringe filter (PTFE, hydrophilic, Millex, Sigma Aldrich™, St. Louis, Missouri, USA) to eliminate any remaining particulate matter and the leftover pellet was disposed of.
Preparation of a methodology blank and the CRM was conducted in the same manner as the samples to make sure that the method did not influence the observed As concentrations or distort the analytical results, safeguarding the accuracy of the analysis.
As part of the sample sequence, a spiked rice sample (5 µg/L of all As species) was analyzed to validate the analytical method for each single As species in the sample.
Preparation of the mobile phase derived from HNO3 (Suzhou JINGRUI Chemical Co. Ltd.), H3PO4 (85%, Fisher Scientific, Hampton, New Hampshire, USA) and NH4OH (≥25%, Sigma Aldrich, Burlington, Massachusetts, USA).
Throughout this work, the calibration standards, rice extract, CRM, and blanks were poured into metal-free polypropylene HPLC vials and evaluated without dilution.
To take into consideration the changing gradient of the chromatographic baseline, a bypass sample was assessed as part of the sequence and deducted from the blank, sample, and CRM chromatograms.
Instrumentation
All analyses were carried out on a NexSAR Speciation Analysis Ready HPLC system (PerkinElmer Inc., Shelton, Connecticut, USA) made up of the NexSAR 200 Inert HPLC Pump, Cooled Inert Autosampler, and Solvent Tray with Degasser. The system was paired with a NexION ICP-MS (PerkinElmer Inc.).
Details relating to the HPLC and ICP-MS conditions are illustrated in Tables 1 and 2, respectively. Samples and standards were performed in standard mode where a correction equation was utilized to compensate for the interference of ArCl+ polyatomic.
All analyses and data acquisition was conducted using Clarity™ chromatography software (DataApex, Prague, The Czech Republic).
Table 1. NexSAR Inert HPLC Conditions. Source: PerkinElmer Food Safety and Quality
| Component/Parameter |
Type/Value |
| Chromatography |
Anion exchange chromatography |
| Mobile Phase |
A: 10 mM ammonium phosphate
B: 10 mM ammonium nitrate |
| pH |
8.6 |
| Flow |
1 mL/min |
| Separation Scheme |
Gradient |
| Run Time |
16 min |
| Injection Volume |
100 μL |
| Column Temperature |
30 °C |
| Injection Type |
Full loop |
| LC Vials |
HPLC tested PP vials, 1.5 mL |
Table 2. NexION ICP-MS Conditions. Source: PerkinElmer Food Safety and Quality
| Component/Parameter |
Type/Value |
| Nebulizer |
MEINHARD® Plus Glass Type C |
| Spray Chamber |
Glass cyclonic |
| RF Power |
1600 W |
| Injector |
2.0 mm I.D. quartz |
| Mode |
Standard |
| Dwell Time |
500 ms |
Results and Discussion
The correlation coefficients for the standards (0.2-100 µg/L, n=6) of AsB, As III, DMA, MMA and As V were 0.999965, 0.999979, 0.999999, 0.999999, and 0.999989, respectively (Figure 1a-e), proving superb linearity across the concentration range expected for As in rice as determined by GB 5009.11.
The overlay of the calibration standards from 0.2-100 µg/L (Figure 2) shows the consistent, reliable, and reproducible flows which the NexSAR 200 Inert HPLC Pump offers. This ensures that peaks are properly identified and are consistently sharp, facilitating and accomplishing excellent accuracy and signal-to-noise (S/N) ratios.
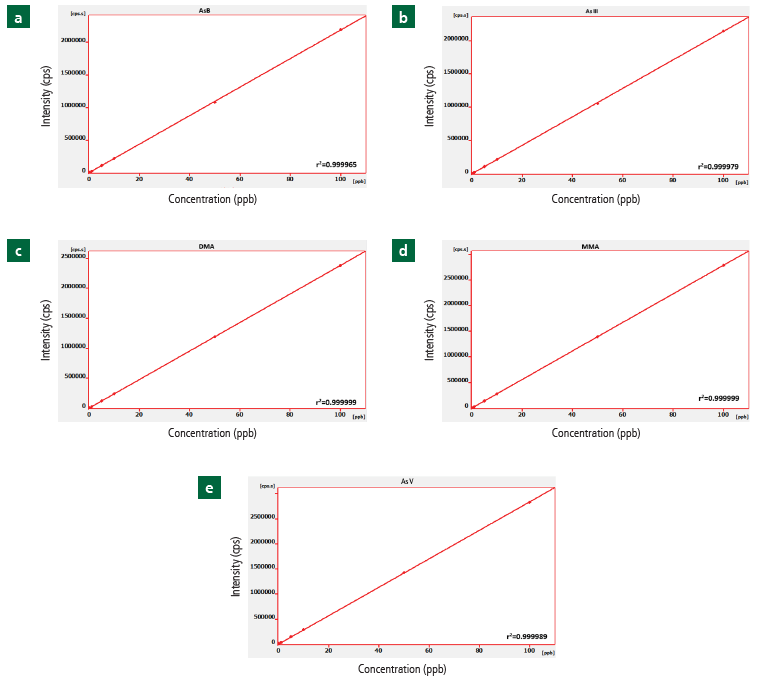
Figure 1. Linear regression of calibration standards ranging in concentration from 0.2-100 μg/L for (a) AsB, (b) As III, (c) DMA, (d) MMA, (e) As V in the mobile phase (pH 8.6) and the respective correlation coefficients. Image Credit: PerkinElmer Food Safety and Quality
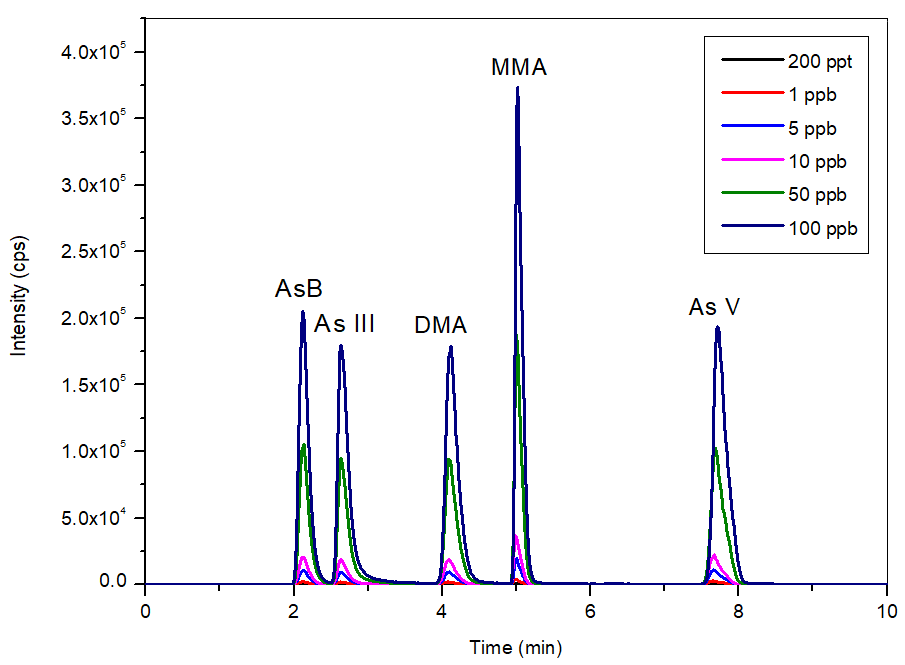
Figure 2. Bypass subtracted overlay of calibration standards (0.2-100 μg/L, n=6) in the mobile phase at pH 8.6. Image Credit: PerkinElmer Food Safety and Quality
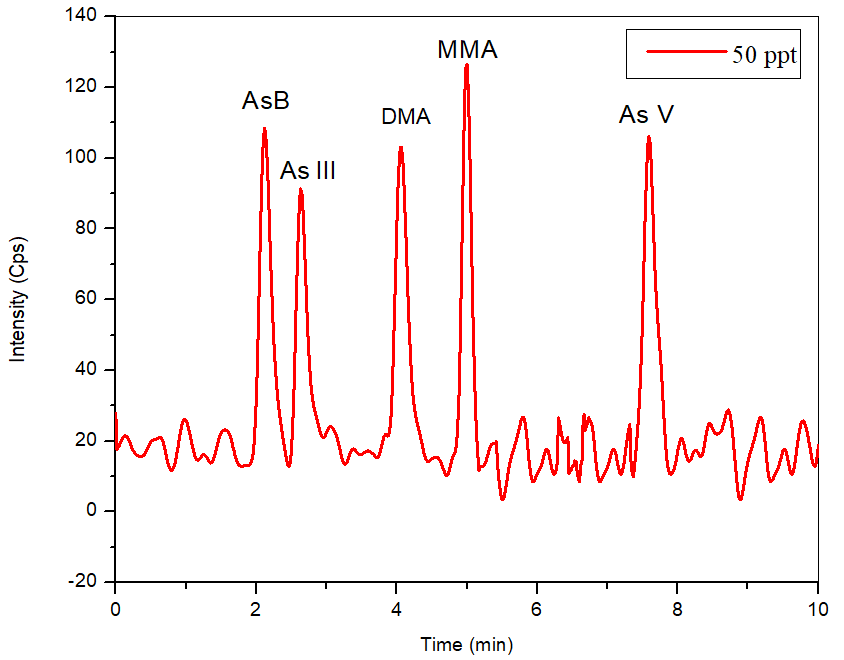
Figure 3. 50 ng/L standard (bypass subtracted) of five species of arsenic to determine the theoretical detection limits. Image Credit: PerkinElmer Food Safety and Quality
The S/N ratio for a 50 ng/L standard (Figure 3) was 15, 13, 13, 17 and 23 for AsB, As III, DMA, MMA, and As V, respectively, utilizing the mobile phase defined, generating a theoretical detection limit of 11.5 ng/L for As III and DMA, 6.5 ng/L for As V, 8.8 ng/L for MMA, and 10 ng/L for AsB.
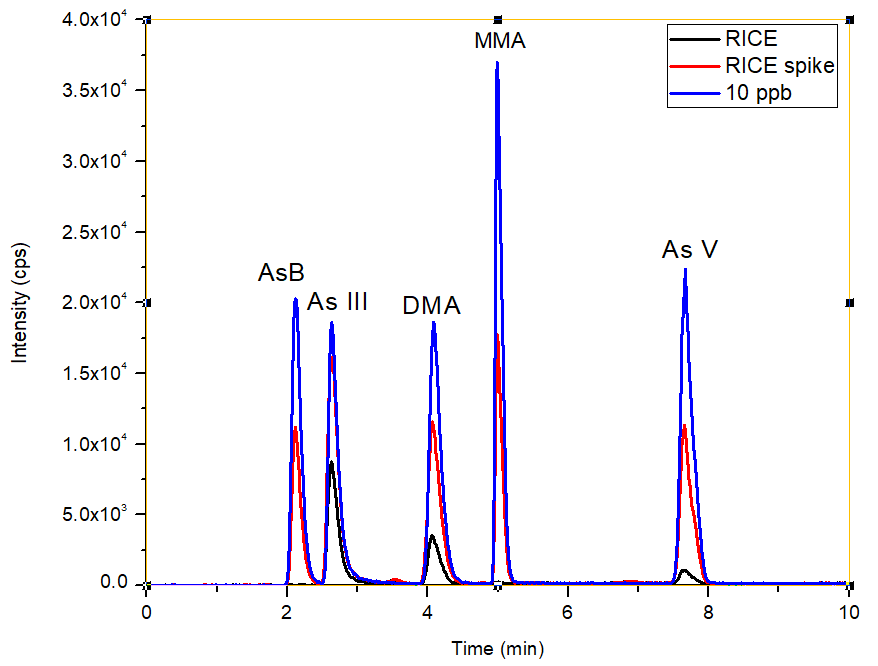
Figure 4. Overlay of chromatograms (bypass subtracted) for the rice sample, spiked rice sample and 10 μg/L standard. Image Credit: PerkinElmer Food Safety and Quality
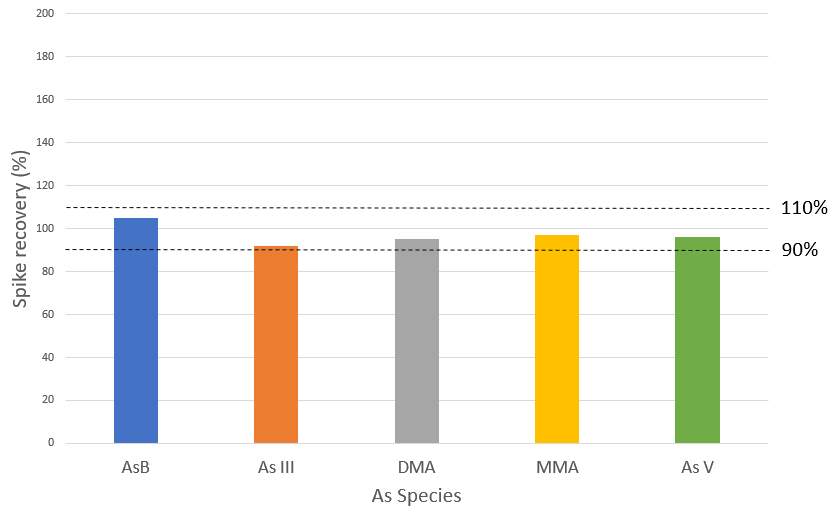
Figure 5. Spike recoveries for 5 μg/L spike of AsB, As III, DMA, MMA and As V in rice extract. Image Credit: PerkinElmer Food Safety and Quality
The overlay of the unspiked rice sample, the spiked (5 µg/L) rice sample, and the 10 µg/L standard is illustrated in Figure 4. This overlay shows that the sample matrix does not possess an effect on the retention time of various As species.
This proves the strength of the method which is improved through the accuracy of the pump flows. Exceptional spike recoveries of 105, 92, 95, 97, and 96% were accomplished for AsB, As III, DMA, MMA, and As V, respectively (Figure 5), which validated further the proven accuracy of the method for each individual As species across the sample matrix.
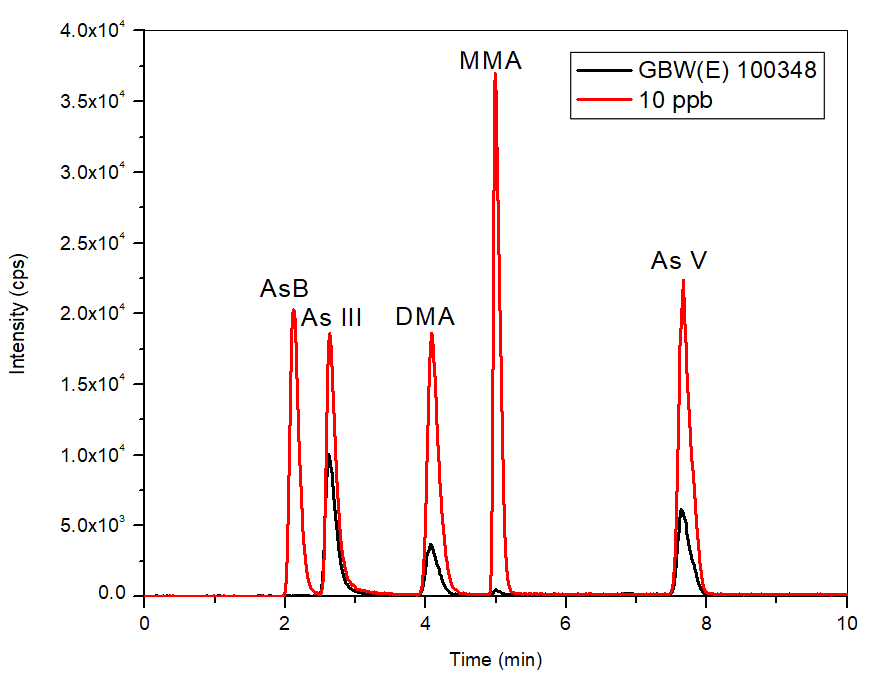
Figure 6. Chromatogram of certified reference material (bypass subtracted) GBW(E) 100348 in comparison to the 10 μg/L standard. Image Credit: PerkinElmer Food Safety and Quality
The method was validated further through the use of a CRM (GBW(E) 100348), where Figure 6 exhibits an overlay of the CRM and the 10 µg/L calibration standard.
The CRM, which possessed the same retention time as the standards, had a 100% recovery for inorganic As and 99% for the total As content, confirming further the analytical precision of the method.
Using the proposed method, the concentration of As species in the rice sample bought from a local store (Table 3) was assessed for the study.
As III, As V and DMA were beyond the method detection limits where the sum of the inorganic As species (As III and As V) were less than the regulated limit of 0.2 mg/kg (GB 2762). AsB and MMA were both lower than the method detection limits and are not observed here.
Table 3. Arsenic Species Concentrations in a Commercial Rice Sample. Source: PerkinElmer Food Safety and Quality
| Species |
Average Concentration ± stdev (μg/g) |
| AsB |
BD* |
| As III |
0.099±0.004 |
| DMA |
0.039±0.001 |
| MMA |
BD* |
| As V |
0.0105±0.001 |
*BD: below detection
Conclusion
This study assessed the As species in a rice sample, spiked rice sample, and rice CRM in accordance with the method proposed by GB 5009.11 by utilizing a NexSAR Inert HPLC system paired with a NexION ICP-MS.
The results clearly showed that low concentrations of As species could be precisely quantified easily using the advised speciation solution and gradient method, where the robust plasma of the NexION and precise flow of the mobile phase provided by the HPLC pump were crucial in being able to accomplish this.
In the commercial rice assessed, the inorganic As content was found to be lower than the regulated amount.
References
- Heikens, A., 2006. Arsenic Contamination of Irrigation Water, Soil and Crops in Bangladesh: Risk Implications for Sustainable Agriculture and Food Safety in Asia. Food and Agricultural Organization of the United Nations, Regional Office for Asia and the Pacific.
- ATSDR, 2013. Arsenic Toxicity: Where is Arsenic Found? https://www.atsdr.cdc.gov/csem/csem.asp?csem=1&po=5
Consumables Used
Table 4. Source: PerkinElmer Food Safety and Quality
| Component |
Description |
Part Number |
| HPLC Vials |
HPLC Tested Plastic Vials, 1.5 mL PP |
N9301736 |
| PEEK Tubing |
Yellow, 0.007” ID, 1/16” OD (5 feet) |
N9302678 |
| PEEK Fittings |
Fingertight for 1/16” OD PEEK Tubing |
09920513 |
Nebulizer
Connector |
PEEK Connection Line for
MEINHARD® Nebulizers |
N8152484 |

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.