 Interview conducted by Olivia FrostFeb 9 2022
Interview conducted by Olivia FrostFeb 9 2022Why is lithium research and development that uses electron microscopy so useful?
Lithium compounds and alloys have very significant applications in the 21st-century world, whether that's lithium-ion batteries used in electric automotive vehicles or mobile electronic devices or, as lightweight structural alloys in the aerospace or automotive industries.
These technologies are of important commercial value, but importantly, further developments and greater adoption are essential when it comes to meeting the energy reduction plans that our governments have set out for the next 10, 20, 30 years.
Electron microscopy is a key characterization tool already in these market segments. This technique is used both in research and development and failure analysis, whether that's analyzing the structure of lithium-ion batteries, looking at the interfaces between the electrodes and the binder and separator or just characterizing the distribution of particles within a cell.
It can also be used to study lightweight structural alloys and reveal the microstructure and the grain orientation present in a material after processing.
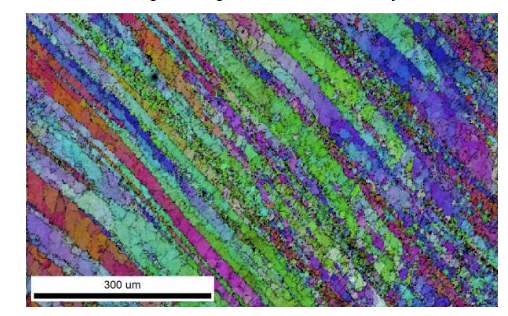
Lightweight structural alloys. EBSD IQ+ orientation map revealing the microstructure of the heat affected zone in a lightweight structure alloy.
Image Credits: EDAX
What are the major challenges that the lithium market faces in terms of innovation?
An inability to map lithium distribution in the scanning electron microscope presents a major obstacle in driving these technologies forward.
Elemental distribution in a material or a device at the microscale is typically determined using a technique called energy dispersive spectroscopy, EDS. In this technique, the electron beam of an SEM generates x-ray fluorescence from a sample. Typically, the energies of those x-rays are characteristic of the atom which produced it, allowing users to determine the elemental composition from the point they have analyzed. By scanning an array of points across a specimen, elemental maps can be generated revealing the elemental distribution within a sample.
The technique has high sensitivity and specificity with sub-micron spatial resolution and can be quantitative.
Although researchers can identify the presence of mamost elements in the periodic table, there remain some key elements whose distribution cannot be observed using EDS e.g., lithium.
It is not just one particular alloy system where this is a problem. In almost all commercially important materials, it is not possible to identify the presence of lithium by EDS as there are no guarantees that lithium x-rays are actually produced by a specimen that contains lithium. The x-ray energy and its fluorescence yield depend on the lithium bonding state and in some materials or compounds, it is just a law of nature, a law of physics, that no lithium x-rays will be generated.
How does this lithium problem extend to SEM?
On the few occasions when lithium x-rays are generated by a sample, they are also readily absorbed prior to reaching the EDS sensor because they have very low energy. This means that they can be readily absorbed by surface contamination, which can be the presence of carbon or hydrocarbons on the surface, or an oxide layer, for example.
Some specialized lithium detectors have been commercialized; however, they have detection limits of about 20 wt.% which can be equivalent to about half the atoms being present in a sample due to lithium's low atomic weight.
Often, the only way that these detectors work to detect lithium is by actually changing the bonding state of the lithium – damaging the specimen to liberate metallic lithium and then analyzing the x-rays generated from the damaged sample.
Can you explain the new approach researchers at the Austrian Institute of Technology have developed to address this lithium challenge?
We developed a brand new method for electrin microscopy based on a composition by difference method; this type of analysis is well accepted in related chemical analysis techniques such as chromatography.
This composition by difference method uses two different signals, which can be quantified, and then compared to determine what is missing.
In this approach, we take a quantified backscattered electron signal, which depends on all elements, to determine the mean atomic number of a region of interest that we're analyzing. From the same region of interest, we also collect the EDS signal. The EDS signal allows us to quantify the non-lithium elements within a sample.
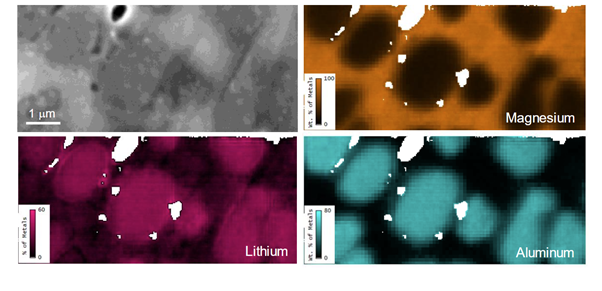
Image Credits: GATAN
How do innovative Gatan and EDAX technology help researchers extract accurate data in this new method?
We have these two different data streams, which we collect using the OnPoint backscattered electron detector from Gatan, and the Octane Elite or Elect Super EDS systems from EDAX. By comparing these two datasets, we are able to extract the lithium content which is missing from the EDS signal.
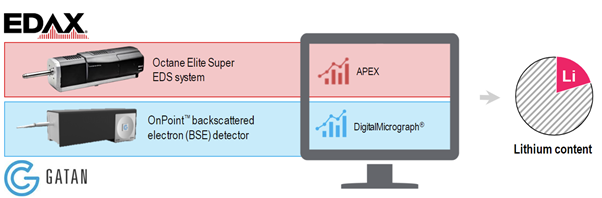
Image Credits: GATAN
Using the composition by difference method, we could quantify single-digit weight percentages of lithium range with good accuracy of approximately 1 wt.%.
The combination of the backscatter electron signal and the quantitative EDS analysis allows us to generate and extract these lithium maps from samples. For example, in a magnesium–lithium–aluminum alloy, we are able to generate single-digit weight percentage maps of lithium mapped quantitatively in the SEM via the composition by difference method using the Octane Elite Super EDS detector and the OnPoint backscattered electron detector.
This is a really exciting development for the characterization of lithium compounds and alloys that will really empower researchers to recharge their lithium research.
Reference: Österreicher, J. A., Simson, C., Großalber, A., Frank, S., & Gneiger, S. (2021). Spatial lithium quantification by backscattered electron microscopy coupled with energy-dispersive X-ray spectroscopy. Scripta Materialia, 194, 113664.
About Dr. Johannes A. Österreicher
Dr. Johannes A. Österreicher is a Senior Scientist at LKR Light Metals Technologies, a subsidiary of the Austrian Institute of Technology. He develops novel methods for scanning electron microscopy to support his work on light metal processing and alloy development. Dr. Österreicher is a member of the International Society for Stereology and Image Analysis (ISSIA) and has won the society’s prestigious PhD competition in 2019. In his free time, he enjoys hiking, rock climbing and other outdoor activities.

This information has been sourced, reviewed and adapted from materials provided by Gatan Inc.
For more information on this source, please visit Gatan Inc.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.