Polydimethylsiloxane (PDMS) is part of a group of polymeric organosilicon compounds generally known as silicones.
Image Credit: Henniker Plasma
PDMS is the most commonly utilized of these compounds and is used in applications ranging from cosmetics and medicines to silly putty. In this article, the use of PDMS in microfluidic device fabrication is discussed.
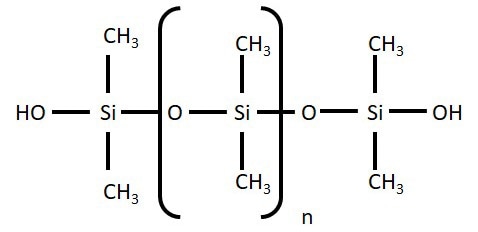
Figure 1. Molecular structure of PDMS. Image Credit: Henniker Plasma
Why PDMS?
PDMS has several properties that make it beneficial for use in forming microfluidic devices. These properties include:
- Low cost
- Low autofluorescence
- Biocompatible
- Transparent (240 nm – 1100 nm range)
- Few nm resolution molding
However, PDMS has one main weakness, which is its poor adhesion to glass. When utilized in the microfluidic application, this may cause premature device failure.
Henniker’s systems are regularly employed for plasma surface treatment of PDMS in microfluidic laboratories globally. These systems enable optimal device bonding results to be achieved.
The Henniker HPT-200 System
The Henniker HPT-200 system is created and optimized for the production of consistent plasma treatment performance for repeatable and reliable bonding of PDMS. Both the PDMS and glass substrates receive treatment with air plasmas at low pressure and with all settings under microprocessor control.

Image Credit: Henniker Plasma
The treatment successfully removes hydrocarbon groups (CxHy) for both substrates, leaving silanol groups remaining on the PDMS and OH groups on the glass substrate, respectively. This enables the formation of strong Si – O – Si covalent bonds between the two materials by the process presented in Figure 2.
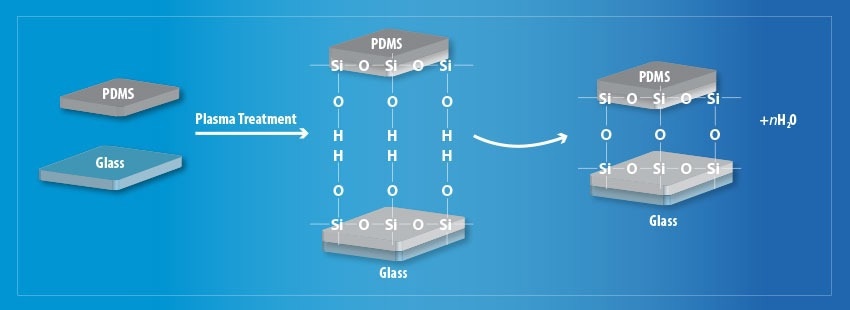
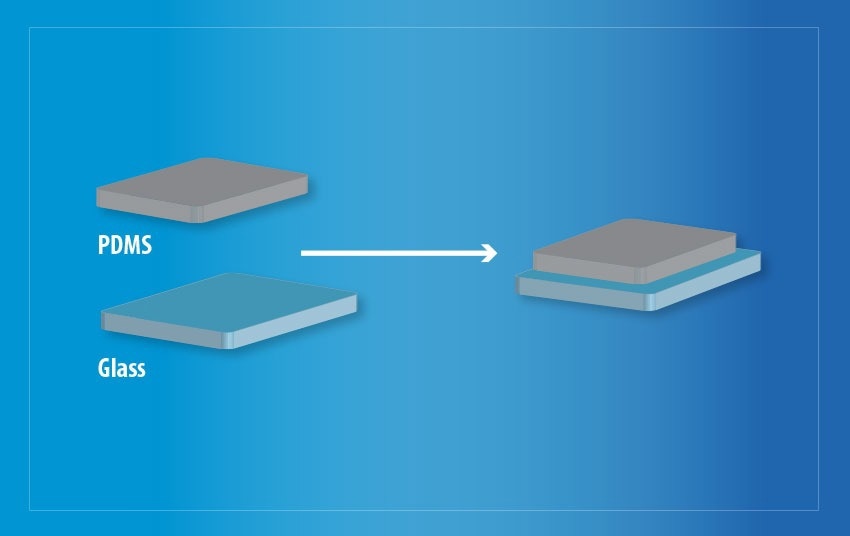
Figure 2. Schematic diagram of the plasma treatment process to improve surface adhesion. Image Credit: Henniker Plasma
Results
1. Contact Angle Measurements
Contact angle measurements reveal whether a surface is hydrophilic (under 90°) or hydrophobic (over 90°) based on the angle formed between a water drop and the surface. Increasing the power or time of an air plasma treatment on PDMS makes the surface more hydrophilic.
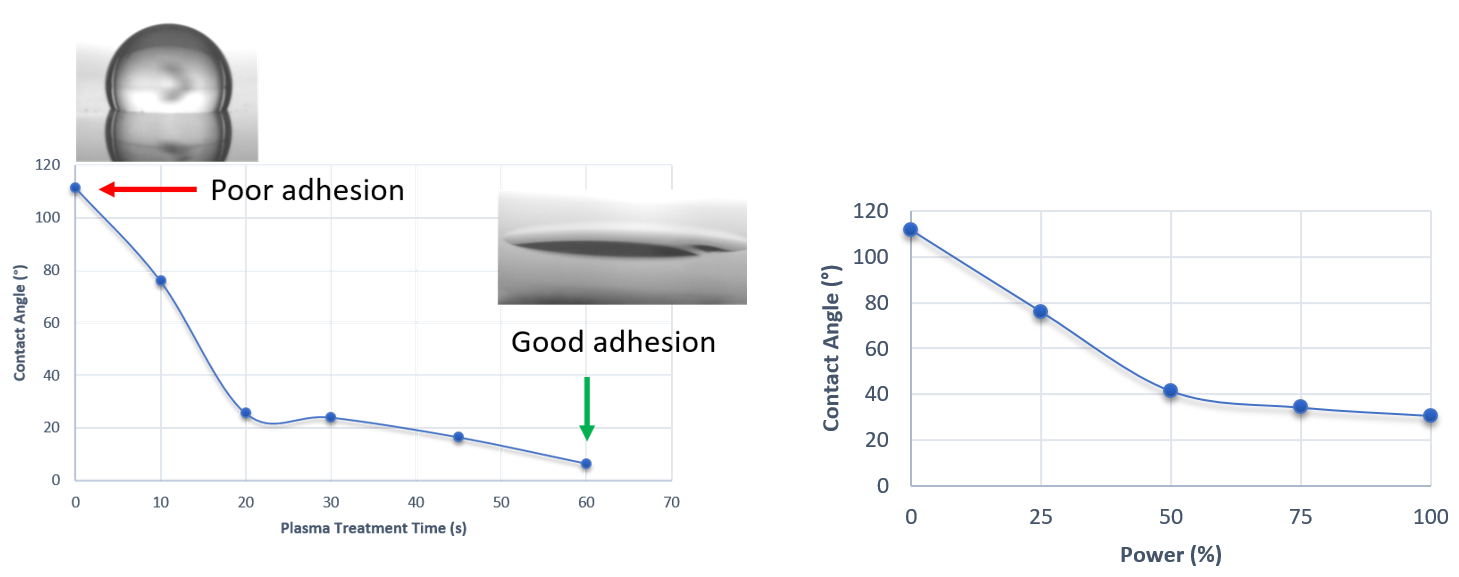
Figure 3. Contact angle variations with increasing treatment time at 25% power (left) and increasing power with 10secs exposures (right). Both show a switch between hydrophobic to hydrophilic behaviour. Insets show example droplets. Image Credit: Henniker Plasma
This swap to more hydrophilic behavior demonstrates that the proposed treatment has been successful and that the –OH termination associated with silanol groups is now exposed, resulting in enhanced bonding of the PDMS to the glass substrates.
2. X-Ray Photoelectron Spectroscopy (XPS)
XPS is a widely utilized method for the analysis of the functional groups present on a substrate’s surface. XPS is employed to exhibit how C-O groups are absent before plasma treatment (in this case, with oxygen plasma) and present after the treatment. This is indicative of a successful surface modification.
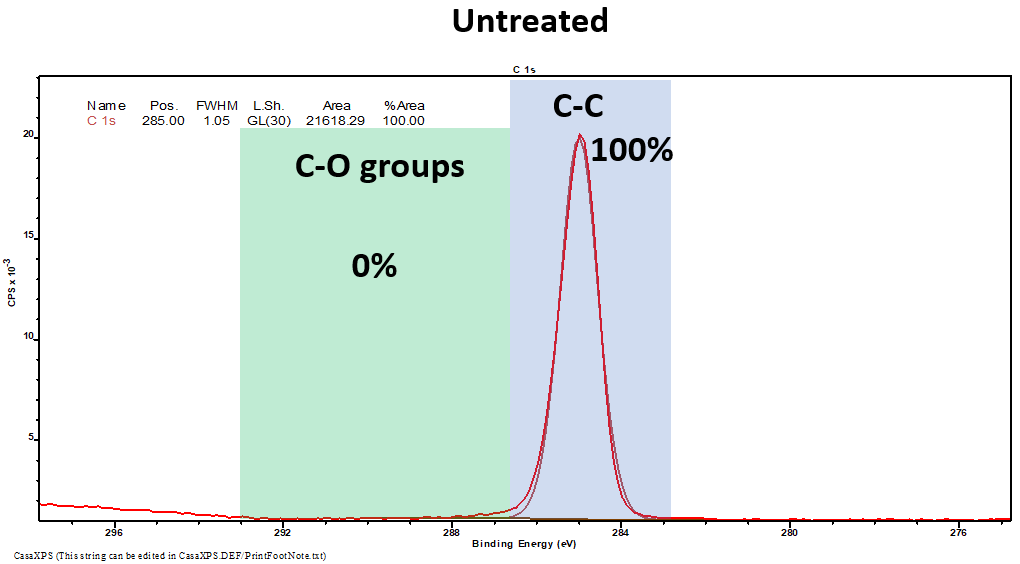
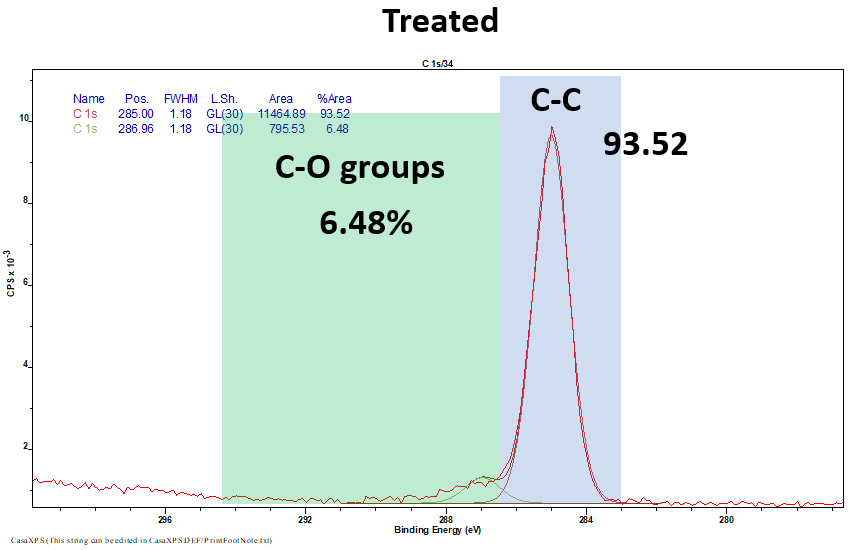
Figure 4. XPS results from untreated (Top) and oxygen-treated (Bottom) samples highlight the presence of C-O groups after treatment. Image Credit: Henniker Plasma
Conclusions
Bonding of PDMS to glass is a key issue in the use of the material in fabricating microfluidic devices. However, this concern can be overcome by using a Henniker model HPT-200 benchtop plasma system to treat PDMS substrates together with glass substrates.
Plasma treatment has been confirmed to enhance the wettability of both surfaces, resulting in increased bonding between the two substrates. This is shown in Figure 6.
The system can also be optimized to bond PDMS to thermoplastic materials.
Figure 5. PDMS microfluidic channels undergoing plasma treatment. Image Credit: Henniker Plasma
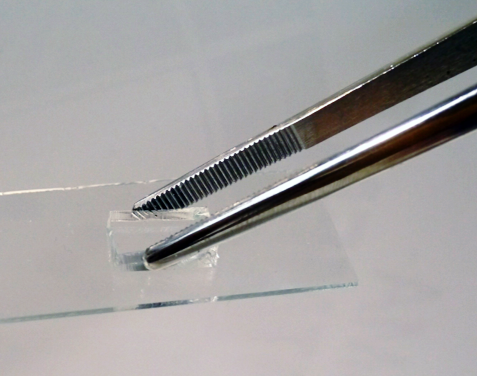
Figure 6. Bonded PDMS and glass following a plasma treatment in a Henniker HPT-200 machine. Image Credit: Henniker Plasma
Acknowledgments
Produced from materials originally authored by Henniker Scientific Ltd. The original authors wish to acknowledge the input and discussion with Dr. Alex Iles of the Pamme Group at Hull University, UK.

This information has been sourced, reviewed and adapted from materials provided by Henniker Plasma.
For more information on this source, please visit Henniker Plasma.