The number of counterfeit medicines is increasing. This used to be considered a problem solely amongst developing countries. However, counterfeit drugs have now also become a threat to the developed world due to increasing numbers of online pharmacies and a demand for affordable medicines.
It is very difficult to trace counterfeit medicines and they offer no guarantee in terms of safety, efficacy or equivalence with the reference product. At best, these medicines are inactive placebos providing nothing but false hope. In the worst-case scenario, such medicines pose a significant risk to health and may even result in severe health problems or death.
The brand image of both regulatory authorities and pharmaceutical companies is being tarnished and profits are dwindling thanks to poor quality counterfeits masquerading under their brand name.

Genuine Tenormin (left) and counterfeit Tenormin (right).
Why Use Raman Imaging for the Identification of Counterfeits?
When compared with conventional techniques such as IR spectroscopy, the clear advantage of Raman is the ability to differentiate between materials of similar chemical structures, even at low concentrations. This specificity makes Raman ideal for the identification of counterfeits, which often use similar components to the reference product.
It is possible to generate high-resolution (1 μm2 per pixel) chemical maps of complex blends of material, for example tablets, with this sensitive technique. Users can study differences in both chemical components and the formulation’s microstructure through these Raman images or ‘maps’.
Identifying Counterfeit Tenormin Tablets
A study was carried out by Renishaw, in collaboration with the Leicester School of Pharmacy, De Montfort University, UK, investigating four different Tenormin tablets sourced from the UK, Saudi Arabia, Nepal and Pakistan.
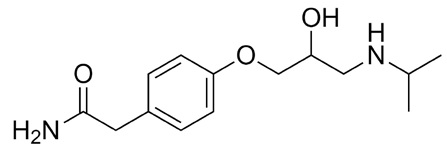
Atenolol (Tenormin) molecular structure.
A brand name of atenolol, Tenormin is a beta-blocker used to treat cardiac disease. If taken in excessive quantities, Tenormin, as with most drugs, is dangerous and an overdose could lead to hypotension-induced shock and acute heart failure.
In order to ensure that the correct dose is consistently delivered to the patient by each tablet in every batch, medicines are required to undergo extensive testing throughout development and manufacturing. There are no such guarantees in terms of safety and efficacy with counterfeit drugs.
Results
Renishaw’s RA802 Pharmaceutical Analyser was used to analyze the four tablets. Thousands of Raman spectra were collected across milled cross-sections of the tablets’ core.

The Renishaw RA802 Pharmaceutical Analyser.
Statistical methods were then applied to analyze the Raman spectra in order to isolate repeating spectral elements – these were assumed to be distinct chemical species. A reference database was then compared against these isolated spectra in order to identify the components within the tablets.
Images of the individual components could then be rendered using the software with a different color used to represent a different formulation component. Particle statistic data was then generated by the software based on the images as well as semi-quantitative data on formulation concentration from an assumed pure contribution from the reference spectra.
Tablet Core
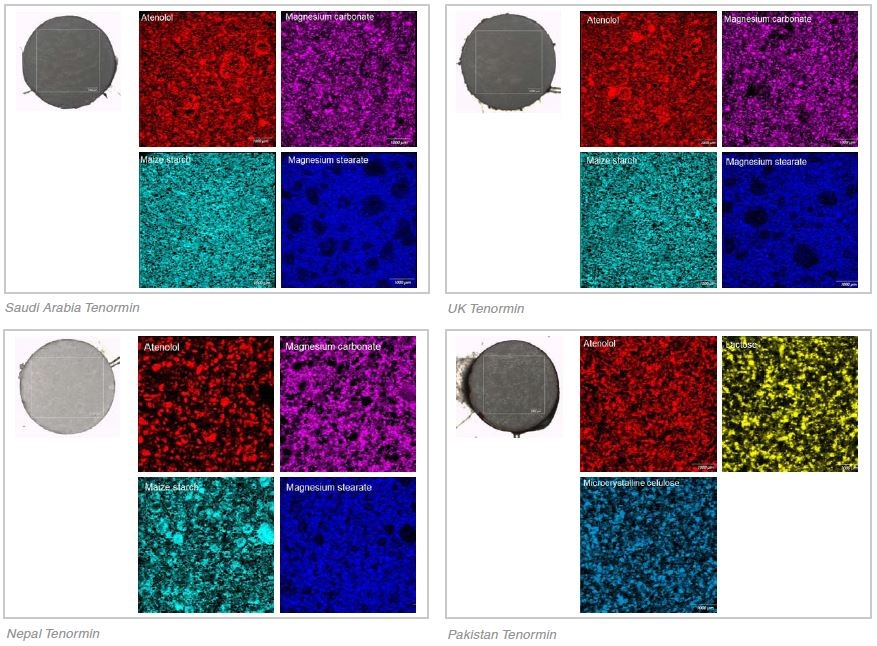
Table 1.
| Tablet Origin |
Components |
Concentration estimate (%) |
| Saudi Arabia |
Atenolol |
77.20 |
| Magnesium carbonate |
15.43 |
| Magnesium stearate |
2.98 |
| Maize starch |
4.38 |
| UK |
Atenolol |
76.47 |
| Magnesium carbonate |
15.40 |
| Magnesium stearate |
3.33 |
| Maize starch |
4.80 |
| Nepal |
Atenolol |
70.34 |
| Magnesium carbonate |
19.51 |
| Magnesium stearate |
4.50 |
| Maize starch |
5.65 |
| Pakistan |
Atenolol |
82.39 |
| Lactose |
2.40 |
| Microcrystalline cellulose |
15.21 |
Tablet Coating
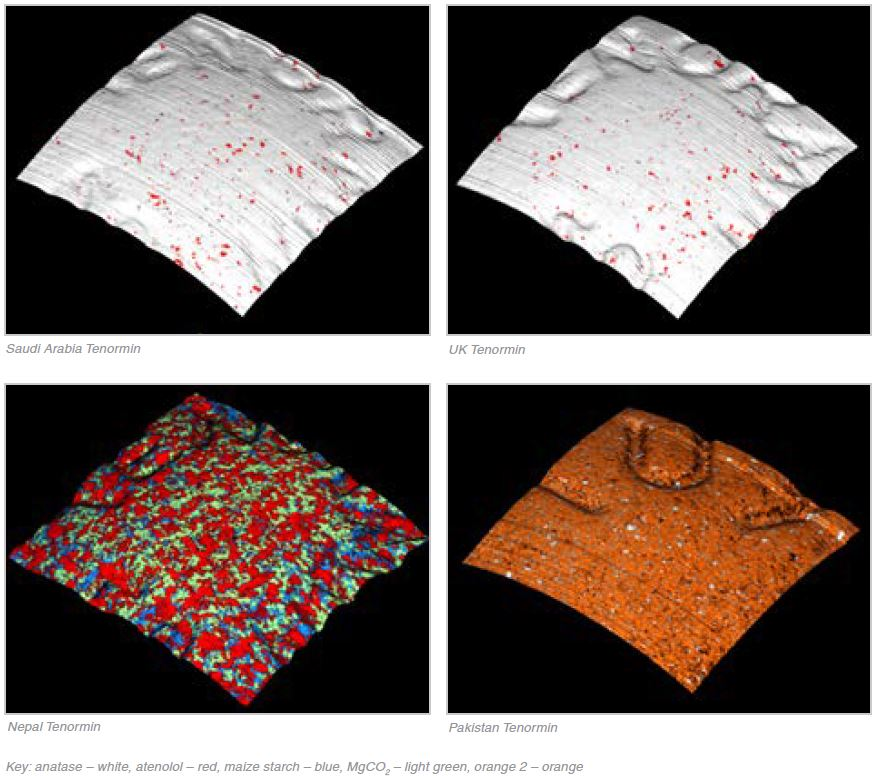
Table 2. Source: Renishaw plc - Spectroscopy
| Identified components |
| Saudi Arabia |
UK |
Nepal |
Pakistan |
| Anatase (titanium dioxide) |
Anatase (titanium dioxide) |
Atenolol |
Anatase |
| Atenolol |
Atenolol |
Magnesium carbonate |
Orange 2 |
| |
|
Maize starch |
|
Discussion
UK and Saudi Arabia
The most similar of the four Tenormin tablets, both qualitatively and quantitatively, were the UK and the Saudi Arabia tablets. As shown in Table 1, the Raman data identified similar concentration of the same components (atenolol, magnesium carbonate, maize starch and magnesium stearate).
The microstructures of the formulations were extremely similar, to the extent that the component images from each of the tablets also looked very similar. The same ingredients, anatase and atenolol, were also contained in the coatings. Whilst the Tenormin from the UK and Saudi Arabia are developed by different manufacturers, these results suggest that both are following the same manufacturing process and both tablets appear to be genuine.
Nepal
The same ingredients are included in the Nepal Tenormin tablet as the UK and Saudi Arabia tablets. However, as shown in Table 1 the concentration estimates of API were approximately 7% lower in the Nepal tablet and there were proportionally higher concentrations of the three excipients - magnesium carbonate, maize starch and magnesium stearate. What’s more, the microstructure was noticeably different to the UK and Saudi Arabia Tenormin.
Whilst the magnesium stearate component looked broadly similar, the atenolol, magnesium carbonate and maize starch components were a lot less well distributed, with large aggregated clumps of material. In contrast, the particle size was much smaller in the UK and Saudi Arabia tablets and they were more uniformly distributed.
The coating, or rather the lack of, was the most obvious indication that this tablet was a counterfeit. The exact same components as the core, minus the magnesium stearate, were contained in the tablet’s surface. This suggests that the powder blend was compressed without any application of a coating. The release of the drug or the decomposition of the drug may be affected by the coating. A complete lack of coating could cause the drug to release differently or lead to the degradation of components, which may result in the patient receiving the incorrect dose.
Pakistan
The most noticeable counterfeit was the Pakistan Tenormin. Only two of the different excipients (lactose and microcrystalline cellulose) were contained in the tablet and neither of these conformed to any of the three excipients contained in the UK, Saudi Arabia and Nepal Tenormin (magnesium carbonate, maize starch and magnesium stearate). As shown in Table 1, the concentration of atenolol was approximately 5% higher than that of the UK/Saudi tablets and a relatively large quantity of disintegrant (15% microcrystalline cellulose) was contained in the Pakistan Tenormin in comparison to the UK/Saudi tablets (≈ 5% maize starch). What’s more the coating of the tablet was predominantly an azo dye, Orange 2, with a minimal amount of anatase.
This tablet would most likely perform differently on dissolution and could exhibit different bioavailability profiles to the reference product, meaning that the wrong dose could be delivered to the patient. The branding of AstraZeneca and ICI were carried on the packaging of the Pakistan Tenormin raising further doubts as to the authenticity of the product.
Conclusions
The seemingly genuine tablets were the UK and Saudi Arabia tablets. The likely counterfeits were identified as the Nepal and Pakistan tablets. The UK and Saudi Arabia Tenormin tablets were highly consistent in terms of qualitative and semi-quantitative data. However, the microstructure differed in the Nepal tablets and the composition differed in the Pakistan tablets. This study demonstrates the possible use of Raman imaging in identifying counterfeit medicines.
Acknowledgments
Renishaw would like to thank Rachel Armitage of Leicester School of Pharmacy, De Montfort University for providing samples and additional information.

This information has been sourced, reviewed and adapted from materials provided by Renishaw plc - Spectroscopy.
For more information on this source, please visit Renishaw plc - Spectroscopy.