Blooms or Bombs?
It is crucial that sufficient crops are produced each year to supply enough food, clothing and other products as the worldwide population increase steadily. Crops like soy, corn, wheat and cotton take in nutrients from the soil they are grown in.

Image Credit: Metrohm AG
Fertilizers play a key part in supplying these crops with the nutrients they require for proper growth. Ammonium nitrate (NH4NO3) is a crucial ingredient in the production of high quality, effective fertilizers. It is a good source of ammonium and nitrogen for plants.
Ammonium nitrate is cheap to buy and usually safe to handle as it is produced as small beads similar in appearance to kitchen salt, but storing it can be challenging. The compound absorbs moisture over time, which results in the clumping of the individual beads into a larger block.

Image Credit: Metrohm AG
An explosion can be triggered when such a large quantity of compacted ammonium nitrate is exposed to intense heat. Ammonium nitrate has been involved in at least 30 disasters and terrorist attacks over the last century.
One of the most recent occurrences and one of the largest industrial disasters ever linked to NH4NO3 was a blast in Beirut on the evening of August 4th, 2020, where an ammonium nitrate explosion killed at least 220 people and injured more than 5000.
Moisture Analysis Methods for Fertilizers
It is crucial to control the moisture content during the production process of ammonium nitrate. A low moisture content is preferable, but unnecessary excess drying results in further manufacturing costs.
Regulations for different fertilizers vary around the world, but local legal limits ensure that the maximum amount of water present must not be exceeded. So, reliable, rapid and accurate techniques for the determination of moisture are needed.
Karl Fischer titration is one of the most common out of those available; oven drying, for example, cannot be utilized with fertilizers that contain ammonium nitrate. Near-infrared spectroscopy (NIRS) provides unique benefits compared to these techniques.
It is a secondary method that produces reliable results within seconds without any sample preparation requirements. NIRS is a non-destructive measurement method and also does not produce any chemical waste.
NIRS Analysis of Solids
The Metrohm DS2500 Solid Analyzer with Large Sample Cup is the most suitable NIR analyzer to measure different parameters in fertilizer or ammonium nitrate pellets.

Image Credit: Metrohm AG
Solid samples (e.g., pellets and granules) which are filled in the rotating DS2500 Large Sample Cup must be placed on the analyzer window. The Large Sample Cup will rotate to compensate for inhomogeneity while scanning the sample.
The sample is illuminated with monochromatic light to keep the energy level as low as possible, as the DS2500 Solid Analyzer is a pre-dispersive system. So, the instrument lid has to be closed before beginning the analysis, so external light does not influence the results.
The NIR radiation comes from below and is partially reflected by the sample to the detector, which is also located underneath the sample vessel plane. The measurement is finished after 45 seconds and a result is shown. This measurement technique is called diffuse reflection, as this reflected light contains all the relevant sample information.
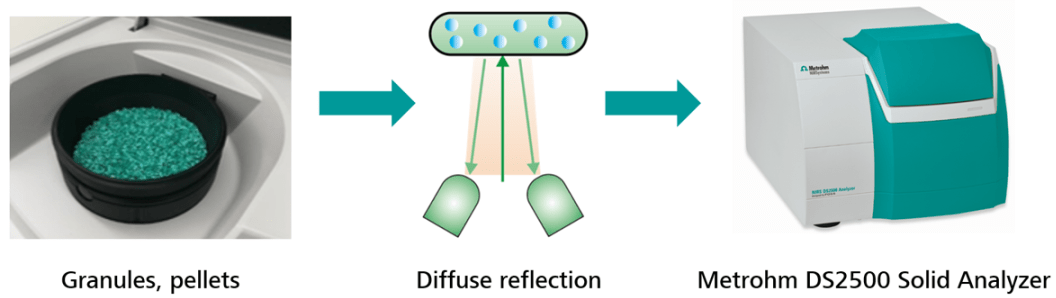
Image Credit: Metrohm AG
Advantages of Using NIRS
The procedure for gathering the NIR spectrum already highlights its simplicity regarding its speed and sample measurement. Numerous benefits of NIRS are listed below:
- Low cost per sample – no solvents or chemicals required.
- Non-destructive – after analysis, precious samples can be reused.
- Fast technique with results in under a minute.
- No sample preparation is needed – liquids and solids can be utilized in pure form.
- Easy to use – inexperienced users are immediately successful.
- Environmentally friendly method – no waste produced.
- Multiple component analysis – prediction of different constituents in parallel.
Overall, near-infrared spectroscopy is a robust alternative method for establishing both physical and chemical parameters in solids and liquids. It is a quick technique that can also be successfully implemented for routine analysis by staff without any higher laboratory education.
Specialty chemicals must fulfill multiple quality requirements. One of these quality parameters is the moisture content, which can be found in almost all certificates of analysis and specifications. Karl Fischer titration is the standard method for the determination of moisture content.
This technique requires reproducible sample preparation, chemicals, and waste disposal. Near-infrared spectroscopy can also be utilized for the determination of moisture content. With this method, samples can be analyzed without using any chemicals and without any preparation.
One of the most commonly measured properties of fertilizers is moisture content. Regulations for different fertilizers vary globally, but local legal limits ensure that the maximum amount of water must not be exceeded. There are various analytical techniques available for this purpose.
Next to gravimetric techniques, Karl Fischer titration is often employed for accurate moisture determination.
Near-infrared spectroscopy provides unique advantages compared to these methods: it produces reliable results within seconds, and at the same time, does not create chemical waste. This article explains how NIRS can provide quick, reagent-free analysis of moisture content in numerous fertilizer products.
Acknowledgments
Produced from materials originally authored by Wim Guns from Metrohm.

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.