May 31 2001
Background
Carbon and chromium can combine to form many different compounds which have uses in welding and thermal spraying. As well as these current applications of chromium carbide, the material has potential as a more environmentally friendly replacement for hard chromium plating.
The Chromium-Carbon System
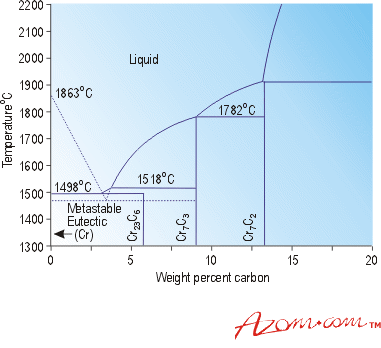
Figure 1 shows a section of the chromium-carbon phase diagram and Table 1 the standard grades of chromium carbide that are produced. As can be seen there are three main compounds and these form the basis of the different grades produced.
|
|
|
Figure 1. Part of the phase diagram for chromium and carbon.
|
Table 1. Standard grades of chromium carbide.
|
|
|
Applications
|
Stick electrodes, flux cored wire
|
Specialist welding and thermal spray
|
Flux cored wire
|
|
Composition
|
(wt%)
|
(wt%)
|
(wt%)
|
|
Cr(min)
|
88
|
85.5
|
79
|
|
C
|
9-11
|
12.8-13.8
|
18-22
|
|
Fe (max)
|
1.00
|
0.50
|
1.00
|
|
Al(max)
|
0.20
|
0.20
|
0.20
|
|
Si(max)
|
0.30
|
0.10
|
0.30
|
|
S(max)
|
0.03
|
0.01
|
0.03
|
|
P(max)
|
0.01
|
0.01
|
0.01
|
Production of Chromium Carbides
London & Scandinavian Metallurgical Co (LSM) produce chromium carbide by a combination of aluminothermic reduction and vacuum furnacing at temperatures of 1500°C and above. A blend of chromium metal, chromium oxide and carbon is prepared and then loaded into a vacuum furnace. The pressure in the furnace is reduced and the temperature increased to 1500°C. The carbon reacts with the chromium oxide to form chromium metal and carbon monoxide gas, which is drawn off into the vacuum pumps. The chromium metal then combines with the remaining carbon to form the chromium carbide.
The exact balance between the chromium oxide, chromium metal and carbon determines the grade of chromium carbide that is produced. This is vigorously controlled to ensure that the product quality is suitable for markets as exacting as aerospace.
Production of Chromium Metal
The chromium metal that is used at LSM s produced by aluminothermic reduction where a blend of chromium oxide and aluminium powder is made up. This is then loaded into a firing vessel where the mixture is ignited. The aluminium reduces the chromium oxide to chromium metal and an alumina slag at temperatures of 2000-2500°C. The chromium forms a molten pool at the bottom of the firing vessel, where it can be collected once the temperature has dropped sufficiently to enable it to be handled. The chromium is then converted to a powder and used as a raw material for the production of chromium carbide.
Grinding of Powders
The grinding of the chromium and the chromium carbide are both carried out in mills. There is always a risk of explosion when grinding fine metal powders and the mills are specially designed to deal with potential hazards such as this. Cryogenic cooling (liquid nitrogen) is also applied to the mill to facilitate grinding.
Applications
Wear Resistant Coatings
Chromium carbides are hard and their general use is to provide hard wear-resistant coatings on parts that need to be protected. When combined with a protective metal matrix, corrosion-resistant as well as wear-resistant coatings can be developed that are easy to apply and cost effective. These coatings are applied by either welding or thermal spray. When combined with other carbides, chromium carbide can be used to form cutting tools.
Welding Electrodes
Chromium carbide welding electrodes are increasingly used instead of the earlier ferrochrome/carbon-containing electrodes, as they give superior and more consistent results. In ferrochrome/carbon-containing welding electrodes, chromium carbide is created during the welding process to provide a hard wear resistant layer. However, the formation of the carbides is determined by the precise conditions in the weld and therefore there can be variation between welds which is not seen with electrodes containing chromium carbide. This is reflected in the wear resistance of the weld deposit. Using the dry sand rubber wheel test, wear rates of welds deposited from ferrochrome/carbon electrodes have been found to be up to 250% greater compared to chromium carbide (table 2).
|
|
|
FeCr/C Weld
|
652
|
2.11x10-10
|
|
CrC Weld
|
753
|
0.82x10-10
|
The trend in the welding industry, which is moving from the use of stick electrodes to flux cored wire is benefiting chromium carbide. Chromium carbide is used almost exclusively in flux cored wire instead of high carbon ferrochrome as it does not suffer from the dilution effect caused by the extra iron in the high carbon ferrochrome. This means that a coating containing a greater number of hard chromium carbide particles can be produced, which exhibits greater wear resistance. Hence, as a switch from stick electrodes to flux cored wire takes place due to the benefits of automation and higher productivity associated with flux cored wire welding technology, the market for chromium carbide increases.
Typical applications for this are the hardfacing of conveyor screws, fuel mixer blades, pump impellers and general applications in which erosive abrasion resistance is required.
Thermal Spray Applications
In thermal spray applications chromium carbide is combined with a metal matrix such as nickel chrome. There is typically a 3:1 ratio by weight of carbide to metal matrix. The metal matrix is present to bond the carbide to the substrate that has been coated and to provide a high degree of corrosion resistance. The combination of corrosion and wear resistance means that the thermally sprayed CrC-NiCr coatings are suitable as a barrier for high temperature wear. It is for this reason that they are finding increasing application in the aerospace market. Typical uses here are as coatings for rod mandrels, hot forming dies, hydraulic valves, machine parts, wear protection of aluminium parts and general applications with good corrosion and abrasion resistance at temperatures up to 700-800°C.
Chrome Plating Alternative
A new application for thermally sprayed coatings is as replacements for hard chrome plating. Hard chrome plating can produce a wear resistant coating with good surface finish at low costs. The chromium coating is obtained by submerging the item to be coated in a tank of chemical solution containing chromium. An electric current is then passed through the tank causing the chromium to deposit onto the part and form a coherent coating. However, there are growing environmental concerns associated with the disposal of the effluents from the used plating solution and these concerns have caused the cost of the process to increase.
Chromium carbide-based coatings have a wear resistance which is between two and a half and five times better than hard chrome plating and do not suffer from effluent disposal problems. They are therefore finding increasing use at the expense of hard chrome plating, particularly if wear resistance is important or if a thick coating is required on a large part. This is an exciting and rapidly growing area which will become more important as the cost of complying with environmental legislation becomes greater.
Cutting Tools
The predominant material in a cutting tool is tungsten carbide powder, which is sintered with cobalt to produce extremely hard cutting tools. In order to improve the toughness of these cutting tools, titanium carbide, niobium carbide and chromium carbide are added to the tungsten carbide. The role of chromium carbide is to prevent grain growth during sintering (a form of grain refinement). Otherwise, excessively large crystals, which would be detrimental to the toughness of the cutting tool, would develop during the sintering process. It is no exaggeration to say that modern cutting tools could not achieve their current performance without the additives.
Primary author: Jonathan Ellis and Michael Haw
Materials World, Vol. 5 No. 11, pp. 136-37, November 1997.
For more information Materials World please visit The Institute of Materials