The steady monitoring of yeast growth and viability is a key element of the fermentation process. The ASBC hemocytometer count technique is the method most frequently used for conducting such observations.
In this method, a hemocytometer is utilized to count samples manually once they have been removed from the fermentation vessel and stained with methylene blue.
While this technique is well-known and widely documented, at best, it is speculation based on a very small sample count. When monitored using a microscope, the hemocytometer shows a grid of measurement areas, as demonstrated below.
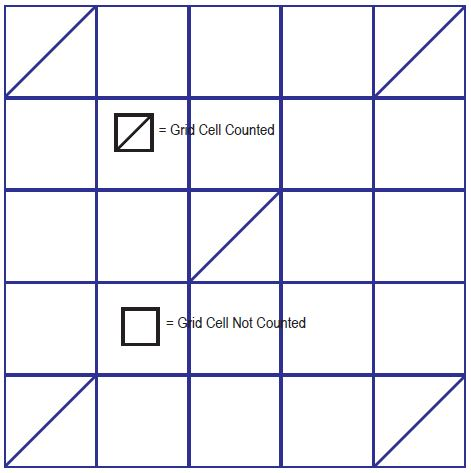
Figure 1. Image Credit: Yokogawa Fluid Imaging Technologies, Inc.
As a result of the time required for the operator to conduct manual counting, only a small number of actual grid cells are counted, which interpolates the results as an average number.
The sample size is extremely small, which produces low statistical significance. It has been determined that errors of up to 25% can be anticipated just by the limited understanding of the operator.
The aim of this article is to present a technique using Flow Imaging Microscopy for enhancing the precision of yeast counts, eradicating the time constraints, and limiting the potential for operator error during this process. Thereby improving the statistical significance by covering a larger sample.
Method
The FlowCam®, a flow imaging microscope, is designed for the optimal automation of this procedure. It can image, count and quantify thousands of individual yeast cells in the time it takes a user to count only tens of cells using the hemocytometer technique.
A count of living, dead and budding yeast cells can be performed automatically by the FlowCam's VisualSpreadsheet® software without the need to involve an operator. This generates extremely precise and repeatable results while eradicating human error. Due to the extensive populations of data captured by the FlowCam, the results have a much greater statistical significance.
As with the hemocytometer technique, the yeast samples are taken out of the fermentation vessel and subsequently prepared by staining them with methylene blue. With the FlowCam in auto image mode, the sample is then processed at seven frames per second as it moves through the flow cell. Each single yeast cell is imaged, saved and quantified as they are taken.
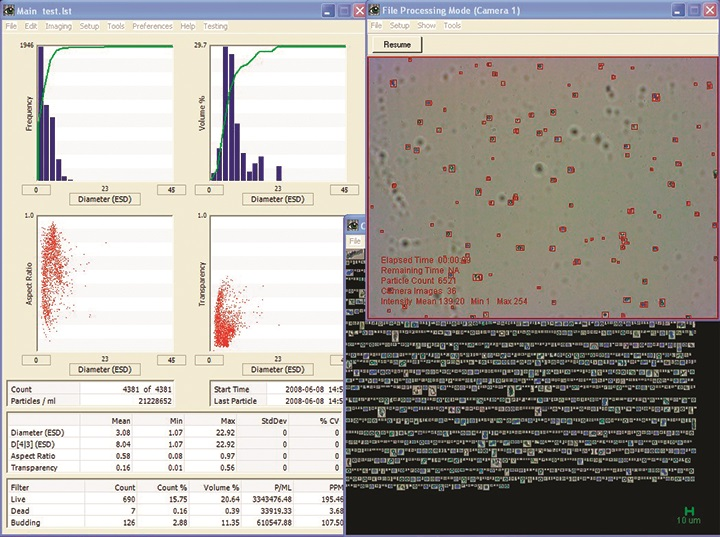
Figure 2. Image Credit: Yokogawa Fluid Imaging Technologies, Inc.
As displayed in Figure 2, the FlowCam instantly captures every yeast cell as a single stored image from the fluid flow. More than 40 morphological characteristics are indexed to the images of singular cells throughout the sample run.
Dead cells will absorb the methylene blue stain, which results in their blue appearance captured by the camera. Figure 3 below shows how the cells would be counted in the hemocytometer.
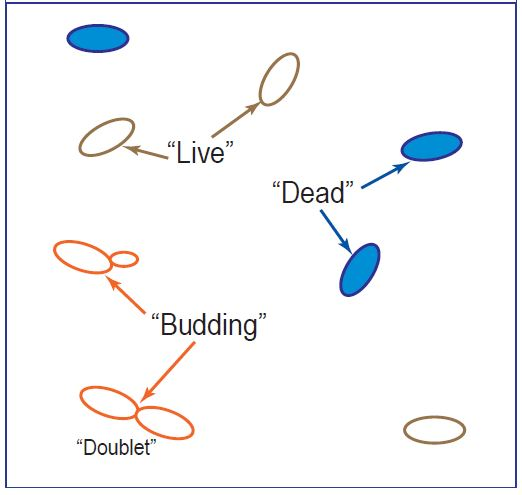
Figure 3. Image Credit: Yokogawa Fluid Imaging Technologies, Inc.
It is easy to distinguish the living from the dead cells using the FlowCam by utilizing the 'average blue' value captured for the cell image (when combined with multiple shape measurements).
A more complicated challenge is put forward by the 'budding' cells due to the fact the resolution needed to appropriately differentiate a single 'live' cell from a 'budding' cell is much greater than what the FlowCam can achieve.
A simple solution to this challenge is to identify 'doublets,' which is when two yeast cells have already 'budded' and will separate. When quantifying 'budding' cells, the operator is primarily searching for signs that the yeast is still growing and tenable.
To quantify budding and find doublets, the system should be filtered and each doublet should be counted as two 'live' cells and one 'budding' cell. In this instance, the trend is the critical measurement as opposed to the actual number.
Results and Conclusions
Figure 4 below exhibits how the FlowCam automatically establishes the quantity of living, dead, and budding yeast cells. In just 35 seconds, the FlowCam automatically classified a total of 8,709 yeast cells during this experiment.
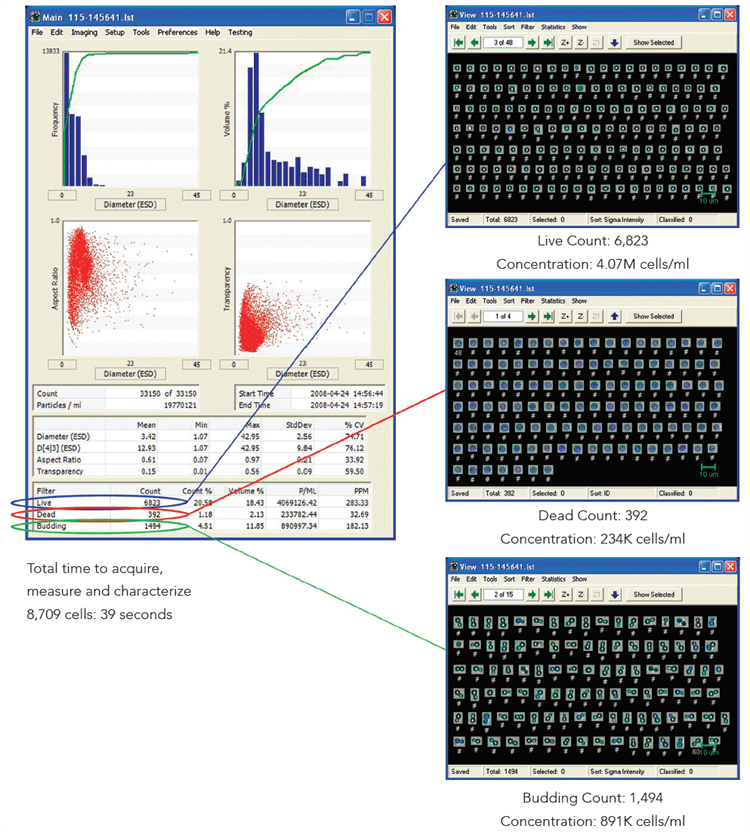
Figure 4. Image Credit: Yokogawa Fluid Imaging Technologies, Inc.
When compared to the hemocytometer counts, the FlowCam performs a precise count of the cells instead of a prediction established on extrapolation. As part of the system, the concentration for each type of cell is calculated automatically by the FlowCam. Due to the large set of data, the results acquired using the FlowCam results offer a greater degree of statistical significance.
As human interpretation errors are eradicated, the FlowCam results produce a high degree of accuracy over multiple runs, with a limited degree of variability, which is typically lower than 1%.
As previously highlighted, the filters used for the characterization of yeast cells only need to be defined once. Filters can be recycled for all subsequent samples once they have been determined.
VisualSpreadsheet allows easy definition of the filters. The user can determine particle images of the required type by pressing on them, instructing the software to save these as a filter.
Utilizing statistical pattern recognition, the filter then easily locates particles that are similar. After this stage, the analysis is entirely automatic.

This information has been sourced, reviewed and adapted from materials provided by Yokogawa Fluid Imaging Technologies, Inc.
For more information on this source, please visit Yokogawa Fluid Imaging Technologies, Inc.