Introduction A biomimetic process modifies a biocompatible material to give it bioactive characteristics [1, 2, 3]. In the case of the implants used for orthopedics or dental applications, such as hip and maxillae facial prostheses, this process allows the deposition of apatite layer on the surface of the implant [1, 2, 4]. Such methods are used to improve the bioactivity of materials such as titanium alloys, tantalum, alumina, and biodegradable polymer composites [5, 6]. This method has shown the following advantages in comparison with the traditional methods [2]: 1) it is a low temperature process that can be applied to any temperature sensitive substrate, 2) it forms apatite crystals, similar to those of bone, showing good bioactivity and good reabsorption characteristics, 3) it can be deposited even in porous substrates or implants of complex geometries, 4) it can incorporate bone growth features. This biomimetic process, in the case of metallic materials, generally consists of a chemical treatment in an alkaline solution, followed by a heat treatment and ending with an immersion in a simulated body fluid (SBF). The immersion in SBF can be considered as a first-stage procedure for the bioactivity assessment of a biocompatible material [6, 7]. Some researchers [7-10] have studied and elucidated the apatite formation mechanism on pure Ti and its alloys, Ta and alumina, finding that once the apatite nuclei are formed, crystals spontaneously start to grow by consuming calcium and phosphorous ions from the surrounding solution. This crystal growth is controlled by the ion transfer through the interface between the substrate and the fluid [11]. For cobalt alloys (Vitallium®) under certain conditions, by using a chemical treatment of NaOH 10M at 60°C for 24 hours, followed by a heat treatment at 600°C, no significatives changes on the samples were reported [6]. After the chemical treatment, deposits of an unknown phase were found and there was no formation of an apatite layer after the immersion in SBF [6]. On the other hand by using a chemical treatment of NaOH 5M at 60°C for 24 hours, followed by a heat treatment at 600°C and immersing the sample in SBF [12, 13] the spontaneous formation of a bone-like apatite layer on this alloy has been reported. However, the apatite formation mechanism for the Co alloys has not fully been understood. This work presents the comparative results of the effect of the SBF concentration on the apatite formation between Ti and Co alloys. Materials and Methods To obtain a Co alloy (ASTM F75), containing approximately 0.018 %wt. C, the investment casting technique was used via two raw materials, a wrought alloy and a powder metallurgy alloy (Carpenter Technology Co). The Ti alloy used was the Ti6Al4V ELI in annealed condition as supplied (Carpenter Technology Co). The cast bars of the cobalt alloy obtained were heat treated at 1224°C for 75 minutes. Tension test were performed for this material (Instron, model 4206) at a crosshead speed of 3 mm/min according to the ASTM E8 standard. The mechanical properties and chemical analysis for the Ti alloy were provided by the supplier. The chemical analysis of the cast cobalt alloy was performed by emission spectrophotometry (Lab S, Spectro Analytical Instruments) and direct combustion with infrared detection for carbon (LECO model CS 244-748-000, Leco Corporation). To apply the biomimetic process, samples of the alloys with dimensions of 12.7 mm in diameter and 2 mm in height were obtained. These samples were ground with silicon carbide papers ranging from 80 to 1200 grit. Finally, the samples were washed with deionised water and ethanol, dried by compressed air and stored in a desiccator before testing. For the chemical treatment the samples were immersed in 5M and 10M NaOH aqueous solutions for Co alloy and for Ti alloy samples, respectively. The samples immersed were kept at a constant temperature of 60°C for 24 hours. After that period, the samples were rinsed with deionised water and dried for 24 hours at 37°C. A couple of samples were kept to further observation. Then, the samples were heat treated at 600°C for 1 hour and cooled down inside the furnace. After the chemical and heat treatments, one couple of samples was taken for further observation. Following the heat treatment, the samples were immersed in an 0.85SBF or in a 1.3SBF solutions. The 0.85SBF and the 1.3SBF were prepared according to the method proposed by Oyane [14]. The ion concentration of the 0.85SBF, SBF, 1.3SBF and that of the human blood plasma are shown in Table 1. Table 1. Ionic concentration. | | | SBF | 142.0 | 5.0 | 1.5 | 2.5 | 147.8 | 4.2 | 1.0 | 0.5 | | 1.5SBF | 213.0 | 7.5 | 2.3 | 3.8 | 223.0 | 6.3 | 1.5 | 0.8 | | Human plasma | 142.0 | 5.0 | 1.5 | 2.5 | 103.0 | 27.0 | 1.0 | | The samples were immersed in SBF solutions (0.85SBF or 1.3SBF) at 37°C for 21 days. During the immersion period, these solutions were renewed each 7 days. At the end of the immersion period the samples were washed gently with deionised water. The samples surfaces were characterized using Scanning Electron Microscopy (SEM; microscope Philips, model XL 30 ESEM), Energy Dispersive X-Ray Analysis (EDX; software Genesis, EDAX) and X-Ray Diffraction (XRD; Diffractometer Xpert Philips, model PW3040). Results and Discussion Chemical Analysis The chemical analysis of the samples and the requirements of the ASTM F75 and F136 standards are shown in Table 2. Both alloys are within the parameters of their respective standards. Table 2. Chemical analysis results [wt %]. | | | ASTM F75 | Bal. | 27 – 30 | 5 - 7 | 1.00 | 1.00 | 1.00 | 0.75 | 0.35 | | CoCrMo | 63.12 | 28.92 | 5.85 | 0.763 | 0.499 | 0.348 | 0.343 | 0.161 | | | | ASTM F136 | Bal. | 5.5 - 6.5 | 3.5 - 4.5 | 0.08 | 0.25 | 0.012 | 0.05 | 0.13 | | Ti6Al4V | Bal. | 6.07 | 4.30 | 0.01 | 0.15 | 0.0024 | 0.009 | 0.13 | Mechanical Properties Table 3 shows the results of the mechanical properties and the requirements of the ASTM F75 and F136 standards. The alloy obtained by the investment casting process satisfied the standard. Table 3. Mechanical properties. | | | ASTM F75 | 655 (min) | --- | 8 (min.) | 450 (min.) | | CoCrMo | 724.5 | 16.6 | 17.8 | 528 | | ASTM F136 | 860 | --- | 10 | 795 | | Ti6Al4V | 986 | --- | 15.25 | 882 | SEM and EDX Before the microscopy characterization the samples were visually analyzed after the chemical and heat treatment. After the chemical treatment a change in color was observed on the surface of the Ti alloy, while the Co alloy samples remained unchanged. After the heat treatment the Ti alloy samples presented an homogeneous layer on its surface and a change in the surface color. The Co alloy samples presented only a visible change in color. Figures 1 and 2 show the SEM images and their respective EDX spectrum for the Ti and Co alloys samples after chemical treatment. 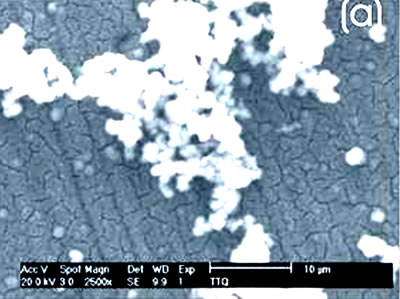
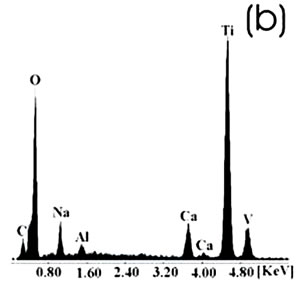
Figure 1. Chemically-treated Ti alloy sample. (a) SEM image and (b) EDX spectrum. 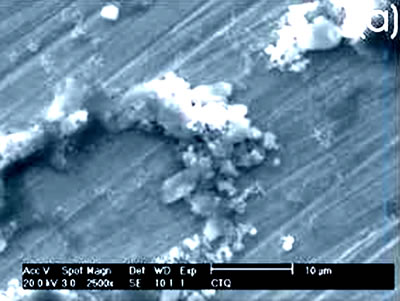
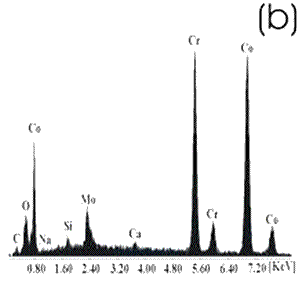
Figure 2. Chemically-treated Co alloy sample. (a) SEM image and (b) EDX spectrum. The chemical treatment modifies more markedly the surface of the Ti alloy than the Co alloys, as reported in the literature [7]. The SEM images show that on the Co alloy the grinding marks are more visible after the chemical treatment, not been the same for the Ti alloys where they have almost disappeared. The chemical treatment forms an alkali titanate hydrogel, which contains some Na or K [1, 4, 9, 10] on the surface of the Ti alloys. For the Co alloys a similar behavior in a certain degree could be expected. However, from the EDX spectrum, as perceived by the surface modification, the Ti alloy sample seems to be more affected by this treatment and the corresponding spectrum presents a more defined and higher Na peak than that in the spectrum for the Co alloy sample. Figures 3 and 4 show the SEM images and their respective EDX spectrum for a Ti alloy and a Co alloy samples with a chemical and heat treatments. 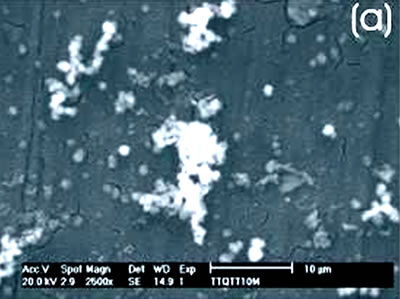
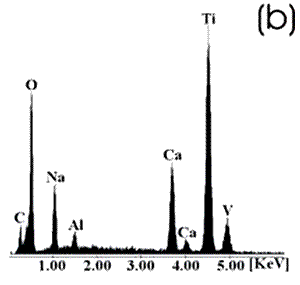
Figure 3. Ti alloy sample with chemical and heat treatments. (a) SEM image and (b) EDX spectrum. 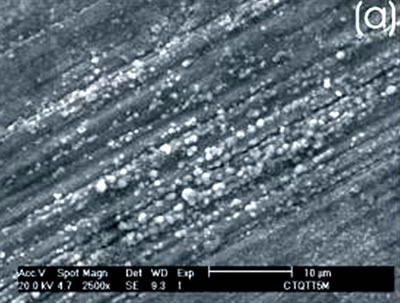
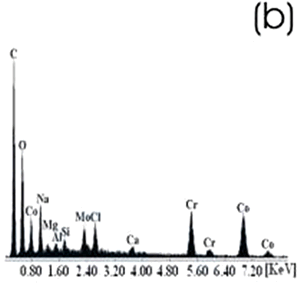
Figure 4. Co alloy sample with chemical and heat treatments. (a) SEM image and (b) EDX spectrum. After the chemical and heat treatments for the Ti alloys the formation of an amorphous alkali titanate in which the Na or K ions are stabilized is reported [1, 4, 9, 10]. Supposing that on the Co alloy a compound containing Na is formed due to the chemical treatment, which may be stabilized during the heat treatment, another surface modification could be expected. From the SEM images these modifications on both alloys are appreciated. Nevertheless, once again for the Ti alloy the effect produced by the two consecutive treatments is more evident than that for the Co alloys, in which the grinding marks are still visible and the change in color is thought to be due to an oxidation mechanism. The EDX spectrum shows that for the Ti alloy the presence of Na is more visible than on the Co alloy, but both exhibit a certain modification due to the previous treatments. Figures 5 and 6 show the SEM images and their respective EDX spectrum for a Ti alloy and a Co alloy samples chemically treated, heat treated and immersed in 0.85SBF for 21 days. | 
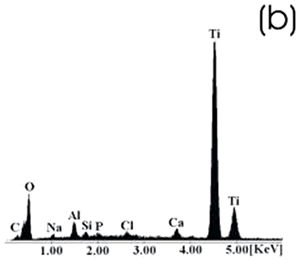
| | Figure 5. Ti alloy sample with chemical treatment, heat treatment and after 21 days of immersion in 0.85SBF. (a) SEM image and (b) EDX spectrum. | | 
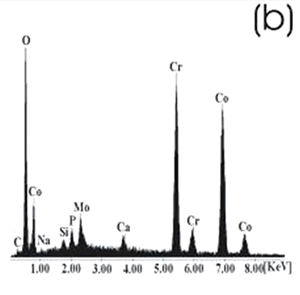
| | Figure 6. Co alloy sample with chemical treatment, heat treatment and after 21 days of immersion in 0.85SBF. (a) SEM image and (b) EDX spectrum. | After the respective treatments and the immersion in 0.85SBF the formation of a ceramic layer, on both samples was observed. However, the layer formed on the Ti alloy seems to be more homogeneous and thicker than that formed on the Co alloy, in which now the grinding marks have almost disappeared. Using the respective alloy peaks on the EDX spectrum, it can be inferred that on both cases the layer is extremely thin. The Ca/P ratios on these samples are shown in Table 4. Table 4. Ca/P ratios for the samples immersed in SBF. | | | Ti6Al4V | 1.4033 | 0.4854 | | CoCrMo | 2.0066 | 0.1285 | The Ca/P ratio range for the apatite is 1.2 – 1.66, for the hydroxyapatite it is 1.67. On the Ti alloy a Ca-deficient apatite seems to be formed, while a compound with a Ca-excess was formed on the Co alloy. Figures 7 and 8 show the SEM images and their respective EDX spectrum for a Ti alloy and a Co alloy samples chemically treated, heat treated and immersed in 1.3SBF for 21 days. | 
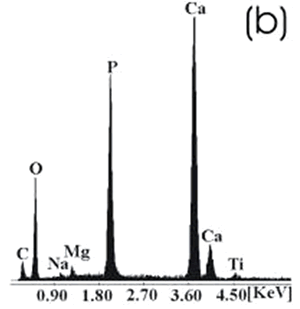
| | Figure 7. Ti alloy sample with chemical treatment, heat treatment and after 21 days of immersion in 1.3SBF. (a) SEM image and (b) EDX spectrum. | | 
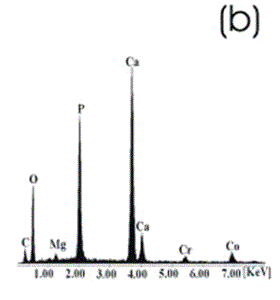
| | Figure 8. Co alloy sample with chemical treatment, heat treatment and after 21 days of immersion in 1.3SBF. (a) SEM image and (b) EDX spectrum. | An apatite layer was observed on both samples. These layers were thicker than those of the samples immersed in 0.85SBF (Figures 5 and 6). Thus, the ionic concentration of the simulated body fluids has a considerable effect on the thickness and composition of the layers formed on the metallic substrates. The Ca/P ratios for the samples immersed in 1.3SBF are shown in Table 5. Table 5. Ca/P ratios for the samples immersed in 1.5SBF. | | | Ti6Al4V | 1.58 | 0.03 | | CoCrMo | 1.49 | 0.0754 | The apatite formed on both alloys was Ca deficient. The layer formed on the Ti alloy showed a closer Ca/P ratio to that of hydroxyapatite. Furthermore, this layer seemed to be thicker and better adhered than that on the Co alloy. The thickness of the layer can be inferred form the EDX spectrum by observing the relative intensity of the peaks for Ca, P, and those of the alloy. The adherence can be inferred from the size and number of cracks observed on the ceramic layers [3,15]. During the treatments of the surface, the surface properties are modified, improving the attachment of the layer formed [3,15]. Once again, the surface modification seems to be more effective on the Ti alloy sample than on the Co alloy sample. X-Ray Diffraction The X-Ray diffraction patterns are given from Figure 9 to Figure 12 for all the samples after the immersion in 0.85SBF or 1.3SBF. For the Ti alloy sample immersed in 0.85SBF (Figure 9) the XRD pattern shows only the corresponding peaks of the Ti alloy. For the Co alloy sample (Figure 10) the result indicated the presence a complex CoCr oxide on the surface. These results may indicate that the layer obtained by the immersion in 0.85SBF is extremely thin, undetectable by XRD. For the Ti alloy (Figure 11) and Co alloy samples (Figure 12), immersed in 1.3SBF, the XRD patterns indicate the presence of hydroxyapatite. A thicker layer was formed when samples were immersed in a 1.3SBF solution. It is possible to observe that the biomimetic method used, consisting of chemical and heat treatments, led to a more modified surface on the Ti alloy than on the Co alloy. After 21 days of immersion in 1.3SBF, the apatite layer formed on the surface of the Ti alloy was thicker than that formed on the Co alloy. However, the Ca/P ratios and morphological characteristics of the layers formed on both alloys are similar. Results obtained when the plasma spray method is used indicate that the apatite layers obtained on the surface of Ti and Co alloys have similar mechanical and histological characteristics and also the same thickness [16]. Regarding the mechanism of formation of the apatite layer on the Co alloy by using the biomimetic method, it is only possible to present two observations: 1) The presence of Na on the surface of the Co alloy sample before the chemical treatment with a NaOH solution is detected, this may indicate the formation of a compound containing Na, and 2) According to thermodynamics, the formation of compounds containing Na, such as chromates, from both chromium or chromite is feasible. However, taking only into account the presence of Na and the thermodynamics it is not possible to elucidate the mechanism of formation of apatite on the Co alloy samples. | 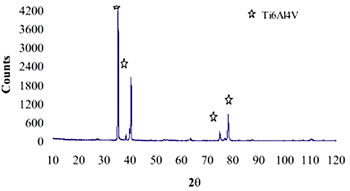
| | Figure 9. XRD pattern of Ti alloy sample with chemical treatment in NaOH 10M, heat treatment and after 21 days of immersion in 0.85SBF. | | 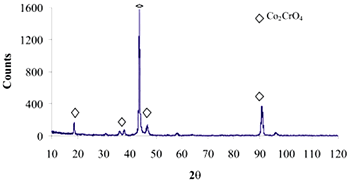
| | Figure 10. XRD pattern of Co alloy sample with chemical treatment in NaOH 5M, heat treatment and after 21 days of immersion in 0.85SBF. | | 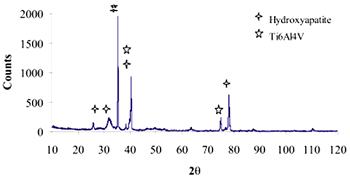
| | Figure 11. XRD pattern of Ti alloy sample with chemical treatment in NaOH 10M, heat treatment and after 21 days of immersion in 1.3SBF. | | 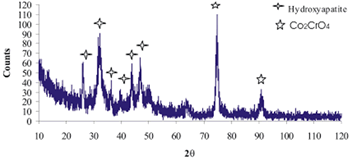
| | Figure 12. XRD pattern of Co alloy sample with chemical treatment in NaOH 5M, heat treatment and after 21 days of immersion in 1.3SBF. | Conclusions As stated in the literature, the formation of an apatite layer on the Ti and Co alloys is possible by using a biomimetic process. The surface modification, due to chemical and heat treatment, was more effective on the Ti alloy samples than that on the Co alloy samples. This surface modification has an effect on the features of the formed layer. To obtain an apatite layer with better characteristics, another way of modifying the surface of the Co alloys must be explored. A Thicker layer of apatite was observed on the alloys immersed in 1.3SBF in comparison with the layer formed on the alloys immersed in 0.85SBF. As the ion concentration on the SBF was increased, the growth rate of the layer was also increased. Acknowledgements The authors would like to thank the Mexican National Council of Science and Technology (CONACyT) for their financial support to this work. References 1. H. Takadama, H.M. Kim, T. Kokubo and T. Nakamura, “TEM-EDX study of mechanism of bone like apatite formation on bioactive titanium metal in simulated body fluid”, J. Biomed. Mater. Res., 57 (2001) 441-448. 2. P. Habibovic, F. Barrère, C. A. van Blitterswijk, K. de Groot and P. Layrolle, “Biomimetic hydroxyapatite coating on metal implants”, J. Am. Ceram. Soc., 85 [3] (2002) 517-522. 3. C. Du, P. Klasens, R. E. Haan, J. Bezemer, F. Z. Cui, K. de Groot and P. Layrolle, “Biomimetic calcium phosphate coatings on Polyactive® 1000/70/30”, J. Biomed. Mater. Res., 59 (2002) 535-546. 4. H. M. Kim, F. Miyaji, T. Kokubo, S. Nishiguchi and T. Nakamura, “Graded surface structure of bioactive titanium prepared by chemical treatment”, J. Biomed. Mater. Res., 45 (1999) 100-107. 5. S. Nishiguchi, H. Kato, H. Fujita, M. Oka, H. Kim, T. Kokubo and T. Nakamura, “Titanium metals form direct bonding to bone after alkali and heat treatments”, Biomaterials, 22 (2001) 2525-2533. 6. F. Miyaji, H.M. Kim, T. Kokubo, T. Kitsugi and T. Nakamura, “Bioactive titanium alloys prepared by chemical surface modification”, Proceedings of the 8th International Symposium on Ceramics in Medicine, 8 (1995) 323-329. 7. F. Miyaji, H.M. Kim, T. Kokubo, T. Kitsugi and T. Nakamura, “Apatite-forming ability of alkali-treated Ti Metal in body enviroment”, J. Ceram. Soc. Jpn., 105 [2] (1997) 111-116. 8. T. Miyazaki, H.M. Kim, F. Miyaji, T. Kokubo, H. Kato and T. Nakamura, “Bioactive tantalum metal prepared by NaOH treatment”, J. Biomed. Mater. Res., 50 (2000) 35-42. 9. H.M. Kim, F. Miyaji, T. Kokubo and T. Nakamura, “Preparation of bioactive Ti and its alloys via simple chemical surface treatment”, J. Biomed. Mater. Res., 32 (1996) 409-417. 10. T. Kokubo, F. Miyaji, H.M. Kim and T. Nakamura, “Spontaneous formation of bonelike apatite layer on chemically treated titanium metals”, J. Am. Ceram. Soc., 79 [4] (1996) 1127-1129. 11. K. Hata and T. Kokubo, “Growth of a bone apatite layer on a substrate by a biomimetic process”, J. Am. Ceram. Soc., 78 [4] (1995) 1049-1053. 12. D. A. Cortés, J. C. Escobedo, A. Nogiwa and R. Muñoz, “Biomimetic hydroxyapatite coating on cobalt based alloys”, Mat. Sci. For., 442 (2003) 61-66. 13. A. Nogiwa, D. A. Cortés, J. C. Escobedo and M. E. Rivas, “Estudio de la formación de apatita en la superficie de aleaciones base cobalto”, Memorias del XXIII Encuentro Nacional de la AMIDIQ. Pátzcuaro, Michoacán, México, April 30-May 3, (2002) 279-280. 14. A. Oyane, K. Onuma, A. Ito, H.M. Kim, T. Kokubo and T. Nakamura, “Formation and growth of clusters in conventional and new kinds of simulated body fluids”, J. Biomed. Mater. Res., 64A (2003) 339-348. 15. M. Tanahashi, T. Yao, T. Kokubo, M. Minoda, T. Miyamoto, T. Nakamura and T. Yamamuro, “Apatite coated on organic polymers by biomimetic process: Improvement in its Adhesion to Substrate by NaOH treatment”, J. Appl. Biomat., 5 (1994) 339-343. 16. R. J. Friedman, T. W. Bauer, K. Garg, M. Jiang, Y. H. An and R. A. Draughn, “Histological and mecanical comparison of hydroxyapatite-coated cobalt-chrome and titanium implants in the rabbit femur”, J. Appl. Biomat., 6 (1995) 231-235. Contact Details |