Introduction
Organic-inorganic hybrid materials are significantly important due to their peculiar properties, which arise from the synergism between the properties of the components [1-4]. The most commonly employed preparation procedure for these materials is the sol-gel process for the formation of the inorganic network, the incorporation of preformed dense inorganic structures, such as clusters and particles and the formation of hybrids using porous of layer inorganic materials [2]. The sol-gel process enables to prepare vitreous and ceramic materials having unique structures and properties rarely obtained by other processing methods. Materials prepared by the sol-gel method are of very high purity and homogeneity and obtained with a lower temperature than those used for other processes [5-7].
The main feature of the hybrid materials is that the organic molecules must be highly dispersed in all the precursor solution in order to get an homogeneous distribution at molecular scale. The most remarkable inorganic material formed by the sol-gel method is unmodified SiO2, using precursors of the type Si(OR)4 for unmodified networks or Rn’ Si(OR)4-n for modified networks [1, 8]. As it is well known the C-Si bond is stable with respect to hydrolysis and then a diversity of modifications can be carried out into the SiO2 network [1]. The main two functions of the organic groups introduced into the sol-gel precursor are: a modification of the inorganic network resulting in improved compatibility between the two phases or the formation of a strong linkage between both phases by means of covalent bonds. Furthermore, the process may be expanded to metal oxide networks using diverse metal alkoxide precursors [9]. Silicon alkoxide as tetraethyl ortosilicate (TEOS) is the most widely used compound to form the inorganic network due to the fact that its hydrolysis rate can be controlled and at the same time allows its copolimerization with other alkyl-substituted silicon alkoxides [10, 11].
On the other hand, many works have demonstrated that hybrid materials can be easily formed by using organic polymers [12, 13]. Therefore, the polymer must contain reactive functional groups such as vinyl, hydroxyl, etc., to react with silanol groups belonging to the hydrolysis of TEOS or be able to react with the hydroxyl groups coming from metallic alkoxides (e.g. Ti, Al, Zr) [14-16]. In fact, metallic alkoxides used in the synthesis of hybrid materials promote the crosslinking of the inorganic network and catalyze the hydrolysis-condensation reactions. Thus, the final properties of the hybrid materials are strongly dependent on the type and concentration of the metallic alkoxide as well as the amount of catalyst, the hydrolysis ratio, the reaction temperature and also the molecular weight of the organic polymer plays an important role in the synthesis [17].
In this work, TEOS and zirconium tetrapropoxide (TPOZ) as inorganic network precursors and polydimethylsiloxane (PDMS) as organic compound were used to obtain different hybrid materials. The effect of TPOZ concentration on structure and texture of the formed materials was mainly studied, maintaining the other synthesis parameters constant.
Experimental Procedure
Materials Synthesis
The hybrid materials were prepared by using TEOS (Merck for analysis) and TPOZ (Aldrich for analysis), and as source of organic part, PDMS (Gelest, Germany) with an average molecular weight of 550. Isopropyl alcohol was used as solvent and hydrochloric acid as catalyst. In this work, the mass ratio of inorganic/organic was kept constant as 70/30. The inorganic part is the sum of TEOS and TPOZ, then, the used amounts of TPOZ (mass ratio) were 0, 3, 5 and 7 and the corresponding amounts of TEOS (mass ratio) were 30, 27, 25 and 23, respectively. The molar ratios of HCl/ inorganic, H2O/ inorganic and solvent/ inorganic were 0.5, 3 and 4.5, respectively. The synthesis of hybrid materials was carried out as follows: three solutions were prepared (A, B and C). The solution A contained TEOS and PDMS. The solution B contained HCl and H2O and finally, the solution C contained the TPOZ. The solvent was added into the three solutions in the same quantity. All solutions were stirred for 1 hour at room temperature. After that time, solution A and solution B were mixed at 80ºC under refluxing and stirring. The solution C was drop-wise added for a half reaction time (30 minutes). A total volume of 300 mL was maintained in all the solutions. After reaction time, the obtained sols were poured into a plastic container allowing gelling. The final materials were dried for 7 days at 50ºC and subsequently at 120ºC. Details concerning to the absolute amounts of reagents used in each preparation are described in Ref. [18].
Characterization techniques
N2 adsorption analysis was carried out in a Tri Star equipment, samples were dried over 12 h in a nitrogen flow. SEM images were obtained in a ZEISS DSM 950 electronic microscopy at 20 kV. The FT-IR spectra were taken with a Perkin Elmer FT-IR spectrophotometer mod. 1720X with a resolution of 2 cm-1 using the KBr pellet method. FT-IR spectra were recorded in the 400-4000 cm-1 spectral range and background was subtracted in all cases.
Results and Discussion
Gelling Time
As mentioned above, the hydrolysis-condensation reactions are controlled by the HCl/inorganic ratio, then, it is an important parameter in the synthesis of hybrid materials. Consequently, the influence of the amount of TPOZ added in the preparations was studied keeping constant the HCl/inorganic ratio of 0.5 in order to investigate modifications on structure, microstructure and texture of the final hybrid materials. In all cases, the obtained gels presented only one phase and were monolithic. Figure 1 shows the gelling times as a function of the amount of TPOZ added in each preparation. As can be seen, the gelling times increased almost exponentially by increasing the amount of TPOZ. These results can be explained in terms of the TPOZ concentration. This means that at our used HCl/inorganic ratio of 0.5, the polycondensation reactions of silanol and Zr-OH groups, generated during the hydrolysis reactions, are inhibited. In other words, a kinetic control on polycondensation reactions is taking place, whereas the hydrolysis reactions are favored under this HCl concentration.
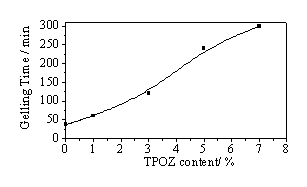
Figure 1. Effect of TPOZ amount on gelling times of hybrid materials.
Texture
Textural properties of the hybrid materials containing TEOS-PDMS-TPOZ were determined by means of the adsorption/desorption nitrogen isotherms and pore size distribution (PSD), pore volume and the specific surface areas were obtained. Figure 2 shows the nitrogen isotherms of the hybrid materials containing different amounts of TPOZ. All isotherms were type IV according to the IUPAC classification and the hysteresis loop Type H3. Typically, if the material under investigation presents a N2 isotherm of type IV accompanied by a type H3 hysteresis loop, then, the solid is purely mesoporous containing non-intersecting mesopores of cylindrical geometry and similar size [19]. However, the hysteresis loop presented in Figure 2 corresponds to type H3 which is the case of more random distribution of pores and interconnected pore systems. As shown in Figure 3, porous materials presented a wide range of pore diameter, between 2 to 60 nm. At higher TPOZ mass ratio (5-7) the accumulated pore volume was lower than that of 1 to 3 mass ratios of TPOZ. In addition, with lower TPOZ mass ratios a clear tendency to ascend of the curves is shown. This means that the formation of micropores is favored at lower mass ratios of TPOZ and then the pore volume is significantly increased. A similar result was found using titanium alkoxide as a precursor of hybrid materials and the microporosity was attributed to the formation of titanium hydroxide nanoparticles partially hydrated [20].
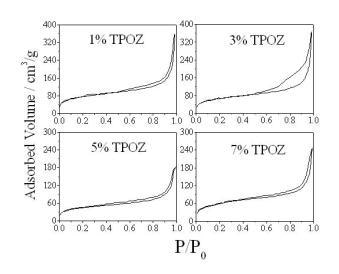
Figure 2. N2 adsorption/desorption isotherms of the hybrid materials containing different amount of TPOZ.
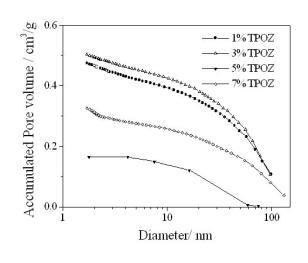
Figure 3. Accumulated pore volume obtained by N2 adsorption containing different amount of TPOZ.
Microstructure
SEM images of hybrid materials with different amount of TPOZ are shown in Figure 4. The microstructure was affected by the amount of TPOZ and all samples showed a different morphology. Sample with 1% TPOZ, Figure 4a, is composed of aggregates with irregular size. However, adding only 3% of TPOZ, Figure 4b, the morphology changed dramatically, it turned smooth with uniform crack direction. The SEM micrograph of sample with 5% TPOZ (Figure 4c) presented a morphology quite similar to that showed in Figure 4a, but the aggregates were smaller and the particle size seems to be less than 1 μm. Finally, in Figure 4d, sample with 7% TPOZ is shown, again, the morphology of this preparation exhibited some peculiarity not seen in the other samples. For instance, an agglomeration of small particles sizing 7-10 μm is clearly seen at the right side of the photograph surrounded by the same conglomeration of fine spherical particles as shown in Figure 4c. The photographs suggest that increasing the amount of TPOZ in the hybrid material, small islands of Zr particles are formed and linked to the inorganic network.
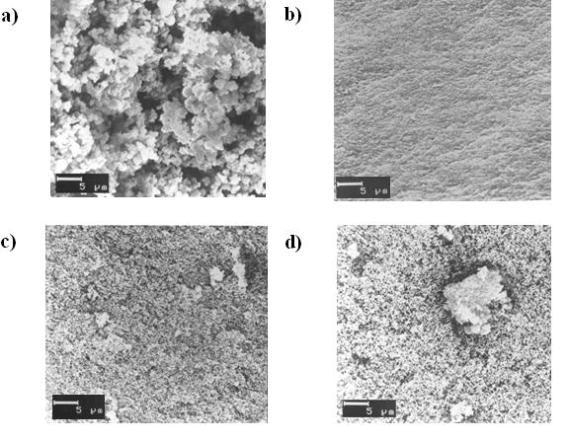
Figure 4. SEM images of hybrid materials containing different amount of TPOZ. a) 1% TPOZ, b) 3% TPOZ, c) 5% TPOZ, d) 7% TPOZ.
Structure
Figure 5 shows the sequence of IR spectra taken in transmission mode for samples containing different amount of TPOZ. All spectra were similar and showed the characteristic bands of vitreous silica [21]. In the region from 1300 to 1000 cm-1 and more specifically at around 1190 cm-1, a shoulder which contains de υa vibrations of Si-O in the Si-O-Si inorganic network, is observed. In addition, an intense wide band at 1090 cm-1 corresponding to silica-gel (υs of Si-O in the Si-O-Si network) is observed. Note that at around 1040 cm-1 appears a shoulder assigned to PDMS accompanied with an intense sharp band at 805 cm-1assigned to υs in SiO4 and υs of Si-C in PDMS. A key information concerning to the incorporation of PDMS (organic polymer) into the inorganic network can be seen at 1263 cm-1 which corresponds to the deforming band of C-H and Si-CH3 bonds. The most significant band to confirm the formation of a hybrid material is that located at 850 cm-1 which is attributed to the copolymerization reaction between TEOS and PDMS in D-Q units (-O-Si-(CH3)2-O-Si-(-O-)3 [22]. At lower frequencies arises a shoulder located at 565 cm-1 assigned to the structural defects in silica glasses and a slight band at 430 cm-1 indicating the deformed vibration of O-Si bond in O-Si-O network [23]. The location as well as width of this band is highly sensitive to the gel structure [24-26] and is dependent of bond angles and the network rigidity. Normally, this band is located at 470 cm-1 and a shift at lower values can be interpreted as a less rigid structure coming from an incomplete network.
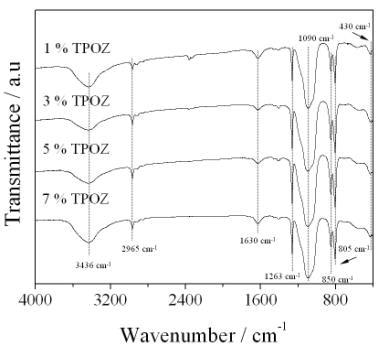
Figure 5. FTIR spectra of hybrid materials containing different amount of TPOZ.
On the other hand, at higher frecuencies between 3800 to 3200 cm-1 a wide band centered at around 3436 cm-1 corresponds to the OH vibration contained in Si-OH groups as well as the H-O-H band. The band located at 1630 cm-1 corresponds to bending vibrations of H-O-H. Finally, the vibration bands at 2965 and 2905 cm-1 are attributed to the υa and υs of C-H in the methyl group of PDMS, respectively. It is important to note that IR analysis did not give evidence of the presence of Si-O-Zr bonds nor those corresponding to the ZrO2. An explanation of this behavior is due to the lower amount of TPOZ used in the synthesis compared with that added for the precursors of the main inorganic network
Conclusions
In this work, amorphous siloxane-zirconia hybrid materials have been successfully prepared by the sol-gel process using TEOS and TPOZ as inorganic precursors and PDMS as organic polymer. In all cases monophasic and monolithic xerogels were obtained. Results showed that increasing the amount of TPOZ an increase of the gelling times was also observed. The final hybrid materials were mainly mesoporous with a wide range of pore diameter average pore distribution around 20 nm and the porosity decreased at higher amounts of TPOZ. Adding TPOZ to the mixture reaction affected significantly the texture and microstructure of the final hybrid materials.
Acknowledgements
This work was supported by a spnish project (MAT2002-03891) of the ministerio of Ciencia y Tecnología. L. Téllez is grateful to the consejo Nacional de Ciencia y Tecnología (CONACYT) under the grant # 72432 and IPN, CGPI Project # 20050911. M.A. Valenzuela thanks CONACYT project # 44118 and COFAA-IPN for financial support.
References
1. G. Kickelbick, “Concepts for the incorporation of inorganic building blocks into organic polymers on a nanaoscale”, Prog. Polym. Sci., Vol. 28, 83-114, 2003.
2. G. Shottner, “Hybrid sol-gel-derived polymers: Applications of multifunctional materials”, Chem. Mater., Vol. 13, 3422-3435, 2001.
3. L. C. Klein (Ed.), “Sol-gel Technology for Thin Films, Fibers, Performs, Electronics and Specialty Shapes”, Noyes Pub., (1988).
4. D. R. Uhlmann and G. Teowee, “Sol-gel Science and Technology: Current state and Future Prospects”, J. Sol-gel Sci. Technol., Vol. 13, 153-192, 1998.
5. H. Schmith and G. Philipp, “New Materials for Contact Lenses prepared from Si an Ti-Alkoxides by the Sol-gel Process”, J. Non-Cryst. Solids, Vol. 63, 283-292, 1984.
6. C. Sanchez and F. Ribot, “Design of Hybrid Organic-Inorganic Materials Synthesized via Sol-gel Chemistry”, New J. Chem., Vol. 18, 1007-1047, 1994.
7. H. Schmidt; “Chemistry of material preparation by the sol-gel process”, Journal of Non-Crystalline Solids, Vol. 100, 51, 1988.
8. U. Schubert, N. Hüsing and A. Lorenz, “Hybrid inorganic-organic materials by sol-gel processing of organofunctional metal alkoxides”, Chem. Mater., Vol. 7, 2010-2027, 1995.
9. H. Schmith, “New Type of Non-Crystalline Solids between Inorganic and Organic Materials”, J. Non-Cryst. Solids, Vol. 73, 681-691, 1985.
10. F. Babonneau, “Hybrid Siloxane-Oxide Materials via Sol-gel Processing: Structural Characterization”, Polyhedron, Vol. 13, 1123-1130, 1994.
11. B. E. Yoldas, “Introduction and Effect of Structural Variations in Inorganic Polymers and Glass Networks”, J. Non-Cryst. Solids, Vol. 51, 105-121, 1982.
12. Y. Hu and J. D. Mackenzie, “Structure-Related Mechanical Properties of Ormosils by Sol-gel Process”, Mater. Res. Soc. Symp. Proc., Vol. 271, 681-685, 1992.
13. G. H. Floch, P. F. Belleville, J. J. Priotton, P. M. Pergon, C. S. Dojonneau and J. Guerain, “Sol-gel Optical Coating for Lasers. I”, Am Ceram. Soc. Bull., Vol. 1, 60-65, 1995.
14. J. D. Mackenzie, Y. J. Chung and Y. Hu, “Rubbery Ormosils and their Applications”, J. Non-Cryst. Solids, Vol. 147&148, 271-279, 1992.
15. S. Diré, F. Babonneau, C. Sanchez and J. Livage, “Sol-gel Synthesis of Siloxane-Oxide Hybrid Coatings”, J. Mater. Chem., Vol. 2, 239-244, 1992.
16. S. Katayama, I. Yoshinaga and N. Yamada, “Síntesis of Inorganic-Organic Hybrids from Metal Alkoxides and Silanol-Terminated Polydimethylsiloxane”, Mater. Res. Soc. Symp. Proc., Vol. 435, 321-326, 1996.
17. C. S. Parkust, W. F. Doyle, L. A. Silverman, S. Singh, M.P. Andersen, D. McClurg, G. E. Wnek and D. R. Uhlmann, “Siloxane Modified SiO2-TiO2 Glasses via Sol-gel”, Mater. Res. Soc. Symp. Proc., Vol. 73, 769-773, 1986.
18. L. Téllez, “Preparación y caracterización de materiales vítreos de Si-C-O reforzados con partículas de carburos metálicos”, Ph. D. thesis, Universidad Autónoma de Madrid (2003).
19. J. C. Groen, L. A. A. Peffer and J. Perez-Ramirez, “Pore size determination in modified-micro and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis”, Microp.Mesop. Mater., Vol. 60, 1-17, 2003.
20. L. Téllez, J. Rubio, M. A. Valenzuela, F. Rubio, E. Morales and J. L. Oteo, “Influence of TiO2 on the Pore Structure and Texture of SiO2-PDMS Hybrid Materials”, Mater. Res. Soc. Symp. Proc., Vol. 847, 183-188, 2005.
21. A. Bertoluzza, C. Fagnano, M. A. Morelli, V. Gotatardi and M. Guglielmi, “Raman an infrared spectra of silica gel evolving toward glasses”, J. Non-Cryst. Solids, Vol. 48, 117, 1982.
22. T. Iwamoto, K. Morita and J. D. Mackenzie, “Liquid State 29Si NMR Study on the Sol-gel Reaction Mechanisms of Ormosils”, J. Non-Cryst. Solids, Vol. 159, 65-72, 1993.
23. Y. Hu, Y.J. Chung, and J. D. Mackenzie, “Gelation kinetics of an organically modified silicate”, J. Mater. Sci., Vol. 28, 6549, 1993.
24. Y. Hoshino, and J. D. Mackenzie; “Viscosity and structure of ormosil solutions”, J. Sol-gel Sci. Technol., Vol. 5, 83, 1995.
25. D. L. Wood and E. M. Rabinovich, “Study of alkoxide silica gels by IR spectroscopy”, Appl. Spectros., Vol. 43, 263, 1989.
26. Y. Hiroyuki, K. Kamiya and H. Nasu, “IR study on the structural evolution of sol-gel derived SiO2 gels in the early stage of conversion to glasses”, J. Non-Cryst. Solids, Vol. 126, 68, 1990.
Contact Details
|