| Virtually everyone has a simple biomaterial in their body. Common tooth fillings represent the first generation of biomaterials, but many people also rely on more critical implants, including joint replacements and cardiovascular implants. Although these have performed successfully, a new generation of biomaterials is emerging that will last longer and be better adapted to prolonged life in the environment of the human body. Hydroxyapatite-Polyethylene Composites This second generation of biomaterials and implants mimic body tissues and provide the basis for both substantially improved surgical procedures and industrial innovation. One such material is hydroxyapatite‑reinforced composite, HAPEXTM, which was pioneered at Queen Mary and Westfield College. It is a composite of hydroxyapatite (HA) in high density polyethylene, which mimics bone, itself a composite of hydroxyapatite in collagen. The HA stiffens the polyethylene, and the polyethylene toughens the composite. Additionally, as bone mineral resembles hydroxyapatite, natural bone will grow onto hydroxyapatite. Design of Biomaterials HAPEXTM highlights the need when designing biomaterials to have knowledge of both materials science and the biological interactions between material and the body. To produce a successful biomaterial which will survive in the body for a long time, materials need to be developed specifically for clinical applications. The primary requirements are biocompatibility, that is the material is not toxic and has appropriate mechanical properties in terms of stiffness and strength. Along with these basic requirements, however, many other factors may need to be included. By choosing the appropriate material, a biological response may be achieved that encourages the surrounding tissue to bond to the implant. It is advantageous to tailor the mechanical properties to match those of the body component which it is replacing, that it is an analogue. The Origins of Biomaterials The earliest biomaterials were sutures, but the significant use of biomaterials as joint replacement implants, or prostheses, began in the UK in the late 1950s when Sir John Charnley developed `low friction arthroplasty'. This changed hip joint replacement from being an occasional major salvage to an almost routine operation. About 40,000 in the UK and half a million in the world are performed each year. Poly(Methymethacrylate) as a Biomaterial Until the 1980s the materials used in joint replacements came from other engineering applications. The grout, or bone cement (poly(methylmethacrylate) - PMMA), used by Sir John Charnley was originally developed for making dentures, but is also suitable for fixing prostheses, and is still used in over 80% of hip and over 90% of knee replacements. Effects of Changes in Society When hip replacements were only performed in the elderly, these materials were successful as the joint replacement outlived the patient. Younger and more active patients are now requiring joint replacements, however, and elderly patients are living longer and consequently suffering from failure of the replacements. The individual components do not fracture, but the biological response of the body to the implants means that they loosen within 5-20 years of the operation, leading to pain and ‘failure’ of the implants. This is a growing problem - in 1995, 18% of all hip replacements in the UK were repeat operations. Natural Bone Figure 1 shows a natural and an artificial hip joint. The bone making up the shaft of the thigh bone is cortical bone whereas the bone foam supporting the joint surfaces at the ends is cancellous bone, which has the same material constituents as cortical bone, but is porous. Most bones are anisotropic and the maximum mechanical properties are in the direction of the maximum stresses. All human bone consists of about 40 vol% (-70 wt%) bone mineral (hydroxyapatite) in a matrix of collagen - a natural composite. | 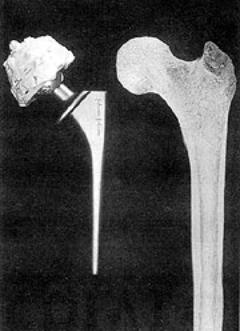
| | Figure 1. Natural and artificial hip joints. | Materials Used in Hip Prostheses In a hip prosthesis, the ball of the natural hip joint is replaced with a metal ball on the end of the stem, which extends down the shaft of the bone. Stainless steel 316L, cobalt chrome alloy, or Ti-6%Al-4%V alloy are normally used. The ball articulates with a hemispherical ultra high molecular weight polyethylene cup. Up to the 1980s, the two components were cemented into the prepared bone using PMMA. More recently, surgeons tried to avoid using PMMA and the femoral implants were inserted as a press fit. Design Evolution of Hip Prostheses This was not very successful, and 50-120µm hydroxyapatite coatings were introduced. The aim of these is to encourage bone to grow up to the implant, and so fix it by direct bone bonding. Other ideas included porous metal coatings to encourage bone ingrowth into the prosthesis. If the mechanical properties are compared, table 1, metals are significantly stiffer than the bone into which they are implanted, whereas polyethylene and PMMA are significantly less stiff. Table 1. Mechanical properties of bone and other materials used in joint replacements. | | | Alumina | 365 | | | | | Hydroxyapatite | 85 | 40-100 | | | | Cobalt-chromium alloy | 230 | 430-1028 | ~100 | ~50000 | | Austenitic stainless steel | 200 | 207-1160 | ~100 | ~50000 | | Ti-6%Al-4%V | 105 | 780-1050 | ~80 | ~10000 | | Cortical Bone | 7-25 | 50-150 | 2-12 | 600-5000 | | Cancellous bone | 0.1-1.0 | | | | | PMMA bone cement | 770 | 1.5 | 400 | | | Polyethylene | 1 | 20-30 | 0.4-40 | ~8000 | The Ideal Biomaterial for Joint Replacement Implanting metals into bone reduces the load on the bone surrounding the implant and, because new bone remodels itself depending on the loads applied to it, bone resorption occurs around the implant which leads to loosening. An ideal material for more successful joint replacements needs similar stiffness, but higher strength compared with cortical bone. It also needs to be bioactive, encouraging bone growth onto the implant. Enter HAPEXTM, and the new generation of biomaterials. Mechanical and Biological Behaviour of HAPEX The mechanical properties of HAPEXTM and the biological response to it have been extensively characterised. In cell culture studies with human osteoblasts (bone making cells), the cells grew and spread over the composite, attaching themselves to hydroxyapatite particles on the surface, figure 2. In vivo testing has shown that a strong and stable interface is developed between the material and the bone into which it is implanted. | 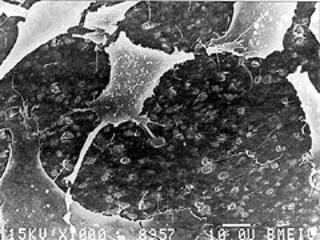
| | Figure 2. Human osteoblast-like cells cultured on the surface of HAPEX. The cells attach themselves to the hydroxyapatite particles. In the composite. | Clinical Behaviour of HAPEX In 1987, two implants made of HAPEXTM were developed to treat clinical problems. The first device was for patients who had fractured their orbital floor - the bone supporting the eye. The second implant was for patients who had lost an eye and had subsequent difficulties in fitting an artificial eye. In both groups of patients, the implants were inserted on the base of the eye socket. They bonded firmly to the supporting bone and none extruded from the eye, unlike the previously-used silicone which was only retained using a soft fibrous capsule. Surgeons found that HAPEXTM could be shaped during the operation using standard tools, unlike bulk hydroxyapatite which needs diamond tipped drills. HAPEX in Middle Ear Implants Following the success of these trials, HAPEXTM has been used for middle ear implants. Sound is transmitted from the outer to inner ear via the three middle ear bones and the vibrations are then translated into electrical signals for processing by the brain. Disruption of the middle ear bones leads to deafness. Various materials have been used for middle ear implants and most extruded from the ear as a result of the body’s response to the material. Hydroxyapatite has successfully been used in contact with the ear drum. Implants have to be trimmed to fit the patient, however, and with all-hydroxyapatite implants, this is extremely difficult. The latest designs have hydroxyapatite heads with HAPEXTM shafts, which may be trimmed to shape using a standard scalpel, figure 3. Following Food and Drug Administration (FDA) approval, trials have shown that this implant was easily shaped in the operating theatre, was good at restoring sound conduction and was tolerated by the body. HAPEXTM was launched commercially in the American Academy of Otolaryngology in September 1995, and in the first year sold over 1000 devices worldwide. | 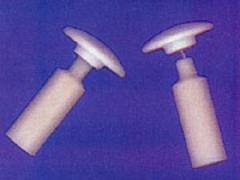
| | Figure 3. Middle ear implants with hydroxyapatite heads and HAPEX shafts. | Summary The Institute of Materials Strategy Commission on Materials Technology Foresight in Biomaterials begins, ‘Biomaterials save lives, relieve suffering and improve the quality of life for a large number of patients’. The Technology Forecast programme on materials also identified biomaterials as a priority area, recommending ‘substantially increased resources (for producing) a new generation of biomaterials which encourage and enhance the restoration and repair of body tissue function’. HAPEXTM – based replacements are the beginning of that generation. |