Silicon nitride etch has been one of the foundations of semiconductor manufacturing for a number of years. Overall silicon (Si) etch rate is dictated by the combination of process temperature and H2O, while selectivity is defined by the Si level.
It is possible to monitor water content through refractive index, conductivity, or non-contact Near Infrared (NIR) spectroscopy – the latter being the most preferred method. A range of commercial analyzers has been designed for this purpose.
Measurement of Si is the main challenge in this process, and an automated method for analysis of Si in traditional Si3N4 etching solution has been outlined previously. High selectivity processes necessitate the use of new solutions, however.
A number of methods have been developed for real-time measurement of the silicon nitride etch process bath. Results from these analyses can be utilized for stringent process control in order to achieve high selectivity for silicon nitride removal.
H3PO4 and H2O Measurement
Both H3PO4 and H2O can be measured using either NIR spectroscopy and conductivity methods. The performance of the two methods is summarized in Table 1.
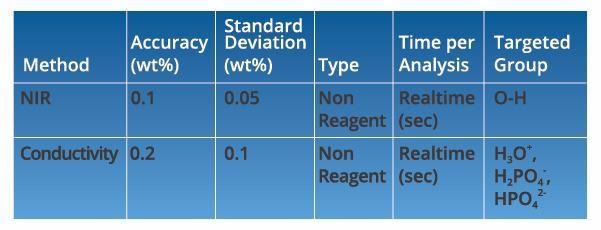
Source: ECI Technology
A comparison of H3PO4 results between an on-line automated NIR method and off-line ICP-MS reveals that on-line results are comparable to ICP-MS results, but with far superior time response (<5 minutes) and the added benefit of automated sampling and feedback. It should be noted that lab analysis via ICP-MS may take several weeks with fab logistics.
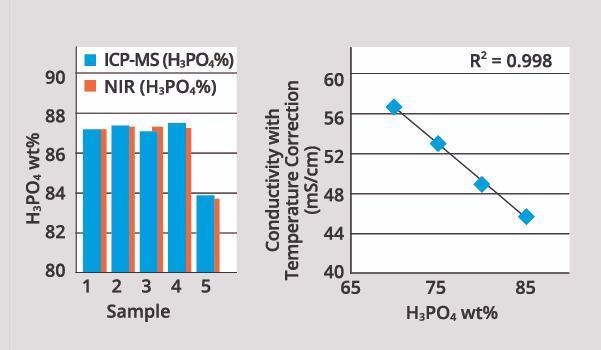
Image Credit: ECI Technology
Conductivity
Conductivity represents ion mobility under the driving force of an electrical field. Conductivity is highly sensitive to temperature, requiring the use of modern temperature control devices to enable efficient temperature control, greatly suppressing the impact of temperature changes.
The figure above displays a characteristic conductivity calibration curve, including temperature correction. This confirms a good correlation with H3PO4 concentration.
Si Measurement
Measurement of silicon is performed via the addition of predetermined concentrations of fluoride ions to a predetermined amount of etchant solution. The potential of a Flouride Ion Specific Electrode (FISE) is measured in this test solution.
In ideal circumstances, the potential (E) of a FISE is given by the established Nernst equation:
E = E0 – (2.303 RT/F) log [F–]
Si Measurement in Low Temperature Etch
Si measurement is performed using a wet bench low temperature hot phosphoric etch process.
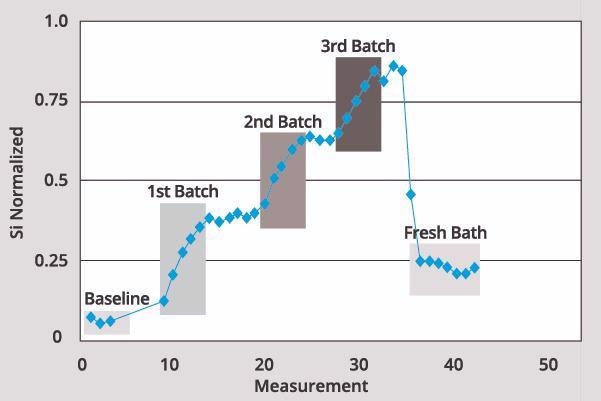
Image Credit: ECI Technology
Organosilicate Measurement
The addition of carboxylic acid to the reagent improves sensitivity. Sensitivity can also be studied with the addition of various fractions of acetic acid included in the reagent.
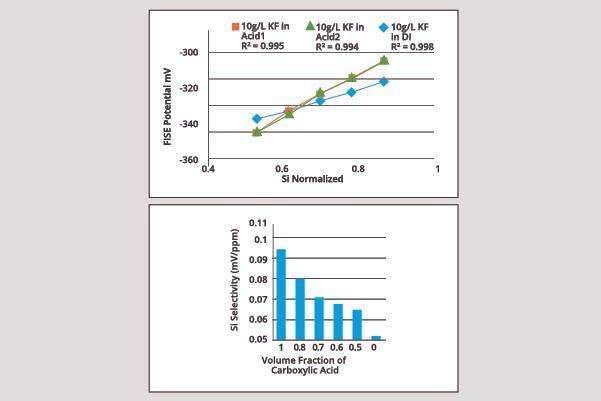
Image Credit: ECI Technology
This method’s accuracy with carboxylic acid in the reagent can be assessed using an off-line ICP-MS method. Results from this improved flouride method have been found to match those from ICP-MS.
Results from the same method with carboxylic acid in the reagent also displayed good stability of organosilicate, with all measured results verified as having accuracy of <2%.
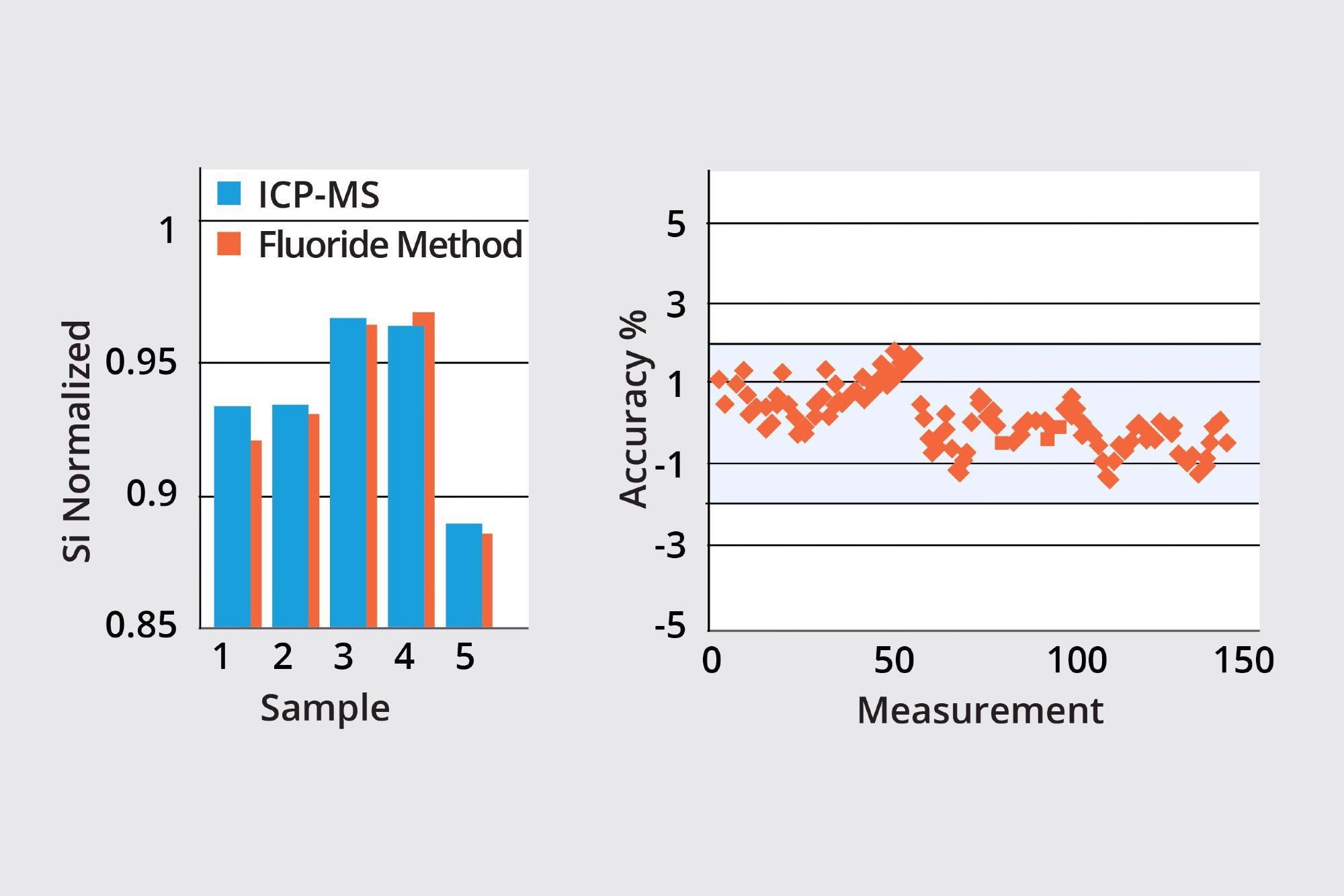
Image Credit: ECI Technology
Conclusions
A range of methods has been developed to enable accurate measurement of the silicon nitride etch process bath in real-time. Results returned from these analyses may be employed in stringent process control in order to achieve high selectivity for silicon nitride removal.
References
- S.J. Baffat, M.S. Lucey, M.R. Yalamanchilli “Hot Phosphoric Acid APC for Silicon Nitride Etch”, Semiconductor International, 8/1/2002
- Hong et al. “Compositions for Etching and Methods of Forming a Semiconductor Device Using the Same”, US Patent 9,136,120
- Cho et al. “Etching Composition and Method for Fabricating Semiconductor Device Using the Same”, US Patent 8,821,752
- Nowling et al. “Low Temperature Etching of Silicon Nitride Structures Using Phosphoric Acid Solutions”, US Patent 8,716,146
- Shalyt et al. “Analysis of Silicon Concentration in Phosphoric Acid Etchant Solutions”, US Patent 8,008,087
- E. Shalyt, G. Liang, P. Bratin, C. Lin “Real-Time Monitoring for Control of Cleaning and Etching Solutions” Proceeds of SPWCC Conference, USA, 2007
- Shalyt et al. “Analysis of Silicon Concentration in Phosphoric Acid Etchant Solutions” US Patent Application 20160018358

This information has been sourced, reviewed and adapted from materials provided by ECI Technology.
For more information on this source, please visit ECI Technology.