Sponsored by Mo-SciApr 15 2021
Controlled pore glass (CPG) is a high silica glass that includes pores with a particular size distribution. Porous glasses can be fabricated into a wide variety of geometric forms (such as beads, frit, plates, rods and hollow spheres), and pore sizes can be tuned precisely from the range of angstroms to millimeters.

Image Credit: Mo-Sci Corporation
Controlled pore glass (CPG) is a high silica glass that includes pores with a particular size distribution. Porous glasses can be fabricated into a wide variety of geometric forms (such as beads, frit, plates, rods and hollow spheres), and pore sizes can be tuned precisely from the range of angstroms to millimeters.
Controlling pore size means that the chemical and physical reactivity of the glass, when exposed to gases and liquids, can be custom-made to suit specific applications, including chromatography, sensing and filtering.
Porous glasses demonstrate high mechanical strength, chemical durability and thermal stability, which make them better suited for a variety of other applications in contrast to other porous media (such as polymers and ceramics).1
This paper identifies how porous glass is fabricated, how pore size can be controlled and some of the various applications of this special material.
Manufacturing Porous Glass
Porous glass can be produced via a number of different routes, each of which produces unique characteristic pore structures. The most frequently used methods involve phase separation or immiscibility of alkali borosilicate glass.
Producing Controlled Pore Glass via the Alkali Borosilicate System
Alkali borosilicate glass systems are made up of a silica glass-former with borate and alkali-oxide additives, which are utilized to reduce the melting temperature of the mixture and transmit other properties.
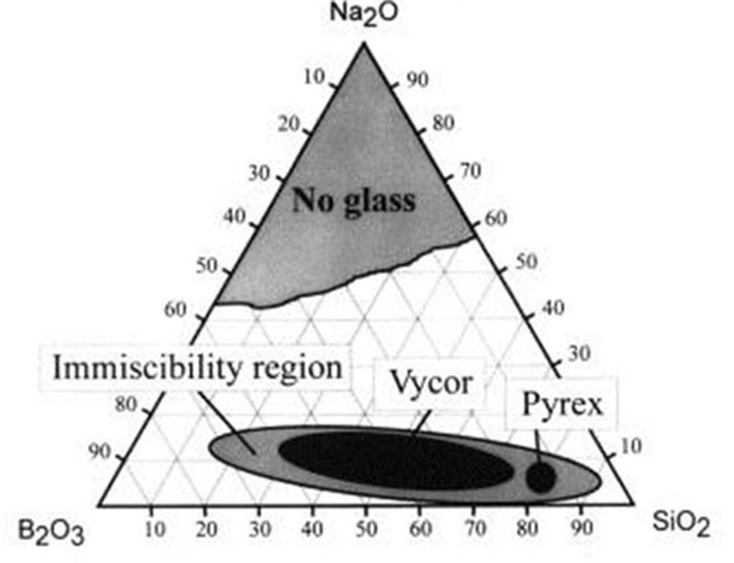
Simplified ternary phase diagram for the Na2O–B2O3–SiO2 system. The “Vycor” region corresponds to the phase separable mixtures that can be used to manufacture porous glass. (Bartl et al., 2001).

Schematic showing the formation of porous glass from a phase separated alkali (sodium) borosilicate mixture. (Hasanuzzaman et al 2016).
In other terms, alkali borosilicate systems are combinations comprised of the chemical species SiO2, B2O3 and R2O, where R is sodium, potassium, or lithium.
When tuning the constituents of this mixture to specific concentrations and subsequently heated, the whole mixture experiences an amorphous phase separation: meaning, the mixture transforms into two distinct phases.
One of these phases is an alkali-rich borate phase, and the other a silica-rich glassy phase. Importantly, the borate phase can be dissolved in acid, while the silica phase cannot. This means that subsequent to heat treatment, a hot-acid solution can be used to leach out the borate phase.
What is left is an exceptionally pure and porous silica glass skeleton with greater surface area: in other words, porous glass.
Controlling Pore Size
Acid-leaching of a phase-separated mixture typically produces an extremely narrow pore size distribution, earning the moniker ‘controlled-pore glass,’ making the resulting glasses appropriate for applications such as adsorptive chromatography of biomolecules.2
As well as glass composition, the typical pore diameter is a function of heat treatment temperature and time. Thus, controlling the heat treatment temperature or time (or both) can produce porous glasses effortlessly with a variety of pore sizes to suit different applications.
Glasses formed using such methods typically have pore diameters in the region of 1 to 1000 nm.3,4
Utilizing alkali borate systems for the formation of porous glass can also be accomplished without inducing a high temperature phase separation: directly etching the surface of the glass can lead to the formation of small pores (1-2 nm) limited to the surface of the glass.
Other Manufacturing Routes
Manufacture of porous glass can also be achieved by glass sintering or via sol-gel routes. Glass sintering is used extensively in the production of glass foams with pore diameters in the region of 400 m to 1 mm.
In sol-gel processes, a solution of organic monomers (sol) is transformed into a glass by extraction of the liquid phase. Sol-gel processes have been successfully used to produce a range of pore sizes for various applications5,6, and they are becoming more frequently used methods.
Applications of Porous Glasses
Porous glass is an alternative to fused quartz which in contrast is difficult to manufacture and form into different geometries. However, a number of emerging applications make use of the functionality the pores themselves offer.
The high surface area and tailorable pore size distribution of these glasses mean porous silica is an exceptionally effective filtering material, with the capacity to separate not only the basis of molecular size but also of molecule type.7
This, along with an extensive range of possible geometries, has made them appropriate for use in biosciences and chemistry.1
For example:
- Enzyme immobilization and size exclusion chromatography methods have been developed using porous glass; making use of its optical transparency, extreme chemical inertness and small pore diameters.5,8,9
- Surface-functionalization of controlled-pore glass using polyaniline has been used in the development of optical chemosensors.10
- Using additives to finely tune the size of pores can lead to functional size-selective catalyst supports.11,12
- The role of porous glass in targeted drug delivery has been studied, utilizing porous-wall hollow glass microspheres. The spheres offer a porous, inert shell for the introduction and release of drugs inside the body.13
- Porous glass is also being evaluated as a bio-scaffold. These applications make use of the strength, porosity, corrosion resistance and biocompatibility of porous glass.14,15
Each of these applications is possible because of the tunability of pore size, which facilitates a set of unique physical properties to be imparted in the glass during the manufacturing process.
Mo-Sci manufactures high purity (> 98% SiO2 and < 2% B2O3) porous glass frit and spheres appropriate across research and industry-led applications. Contact Mo-Sci and speak with an expert concerning your project requirements.
References
- Hasanuzzaman, M., Rafferty, A., Sajjia, M. & Olabi, A.-G. Production and Treatment of Porous Glass Materials for Advanced Usage. in Reference Module in Materials Science and Materials Engineering (Elsevier, 2016). doi:10.1016/b978-0-12-803581-8.03999-0
- Elmer, T. H. Porous and Reconstructed Glasses. in Engineered Materials Handbook (1992).
- Zhu, B. et al. Synthesis and Applications of Porous Glass. J. Shanghai Jiaotong Univ. 24, 681–698 (2019).
- Enke, D., Janowski, F. & Schwieger, W. Porous glasses in the 21st century-a short review. Microporous Mesoporous Mater. 60, 19–30 (2003).
- Lubda, D., Cabrera, K., Nakanishi, K. & Minakuchi, H. SOL-GEL PRODUCTS NEWS Monolithic HPLC Silica Columns. Journal of Sol-Gel Science and Technology 23, (2002).
- Baino, F., Fiume, E., Miola, M. & Verné, E. Bioactive sol-gel glasses: Processing, properties, and applications. Int. J. Appl. Ceram. Technol. 15, 841–860 (2018).
- Hammel, J. J. & Allersma, T. United States Patent | Thermally stable and crush resistant microporous glass catalyst supports and methods of making. 923, 341 (1975).
- Du, W. F., Kuraoka, K., Akai, T. & Yazawa, T. Effect of additive ZrO2 on spinodal phase separation and pore distribution of borosilicate glasses. J. Phys. Chem. B 105, 11949–11954 (2001).
- Jungbauer, A. Chromatographic media for bioseparation. Journal of Chromatography A 1065, 3–12 (2005).
- Sotomayor, P. T. et al. Construction and evaluation of an optical pH sensor based on polyaniline-porous Vycor glass nanocomposite. in Sensors and Actuators, B: Chemical 74, 157–162 (2001).
- Takahashi, T., Yanagimoto, Y., Matsuoka, T. & Kai, T. Hydrogenation activity of benzenes on nickel catalysts supported on porous glass prepared from borosilicate glass with small amounts of metal oxides. Microporous Mater. 6, 189–194 (1996).
- Gronchi, P., Kaddouri, A., Centola, P. & Del Rosso, R. Synthesis of nickel supported catalysts for hydrogen production by sol-gel method. in Journal of Sol-Gel Science and Technology 26, 843–846 (Springer, 2003).
- Using Porous Glass Microspheres for Targeted Drug Delivery Mo-Sci Corporation. Available at: https://mo-sci.com/porous-glass-microsphers-targeted-drug-delivery/. (Accessed: 2nd September 2020)
- Rahaman, M. N. et al. Bioactive glass in tissue engineering. Acta Biomater. 7, 2355–2373 (2011).
- Fu, Q., Saiz, E. & Tomsia, A. P. Bioinspired strong and highly porous glass scaffolds. Adv. Funct. Mater. 21, 1058–1063 (2011).

This information has been sourced, reviewed and adapted from materials provided by Mo-Sci Corp.
For more information on this source, please visit Mo-Sci Corp.