Many individuals and organizations consider nanotechnology to be the next technical revolution, with the potential to drastically change the modern world.
Developments to date may not meet these expectations, but substantial investment from universities, industry and global governments currently exceeds $9 billion annually and is continuing to grow.1 There has been an investment of $27 billion from the United States Government alone between 2001 and 2019.2
Depending on which sources and definitions have been used, the global nanotechnology market has been estimated to be between $10 and $50 billion annually.3,4
Applications of nanotechnology are diverse, though many of these involve the manipulation of matter at the atomic scale. The United States National Nanotechnology Initiative2 defines nanotechnology as the manipulation of matter whereby at least one dimension is between 1 and 100 nanometers (nm).
While nanoparticles remain a key focus of both industrial products and research, the scope of technologies understood to be nanotechnology has widened considerably, now including fields such as microelectronics and Micro-Electro-Mechanical Systems (MEMS).
This article helps to define nanoparticles, outlining their numerous applications and exploring the analytical techniques employed in their physical characterization.
What are Nanoparticles?
A range of governmental bodies and standards organizations have offered agreed-upon definitions of what a nanoparticle is.5,6,7,8,9,10 These include:
“A term referring to a wide range of technologies that measure, manipulate, or incorporate materials and/or features with at least one dimension between approximately 1 and 100 nanometers (nm). Such applications exploit the properties, distinct from bulk/macroscopic systems, of nanoscale components.”5
The European Union’s (EU) Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) provides a more comprehensive definition11 that specifically addresses potential toxicology issues linked to a subset of nanomaterials.
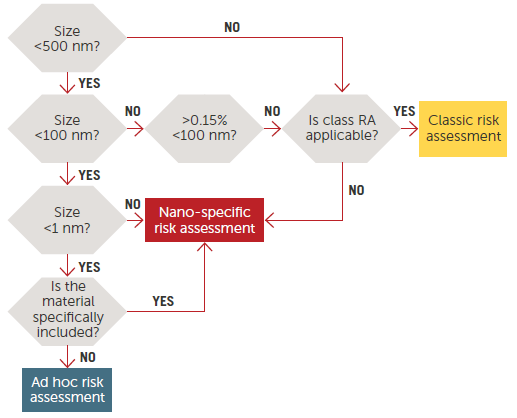
Figure 1. Risk Assessment on nanomaterials, tiered approach.11 Image Credit: Entegris
Figure 1 illustrates a decision tree commonly used to establish three categories of materials and related risk assessments according to size scale.
A number of countries utilize specific definitions which are slightly different from other accepted documentation. These include Taiwan, China, Australia, Canada, France, Switzerland and more.
The Food and Drug Administration (FDA) in the United States provides a Guidance for Industry document12, which covers nanomaterials. In this document, the FDA has opted not to provide strict regulatory definitions of nanotechnology, nanoscale, nanomaterial, or other related terms.
Rather, it outlines current processes used by the FDA when deciding whether or not FDA-regulated products involve nanotechnology applications.
Other Terms
The terms nanotube, nanorod, nanofiber, or nanoplate are occasionally employed in cases where the longest to shortest lengths differ considerably. The term ‘nanostructured materials’ may also be used in cases where nanoscale surfaces or regions exist as part of a material with larger external dimensions.
Analysis Methods
Key nanoparticle physical parameters include shape, size, and surface properties (including dispersion state, charge and crystallinity). A diverse array of analytical techniques is utilized in the quantification of these properties.
Microscopic Techniques
Microscopy remains the most direct size and shape analysis technique. A range of microscopic techniques can be applied to the investigation of nanoparticles; for example:
- Scanning Electron Microscopy (SEM) (Figure 2)
- Scanning Tunneling Microcopy (STM)
- Transmission Electron Microscopy (TEM)
- Atomic Force Microscopy (AFM)
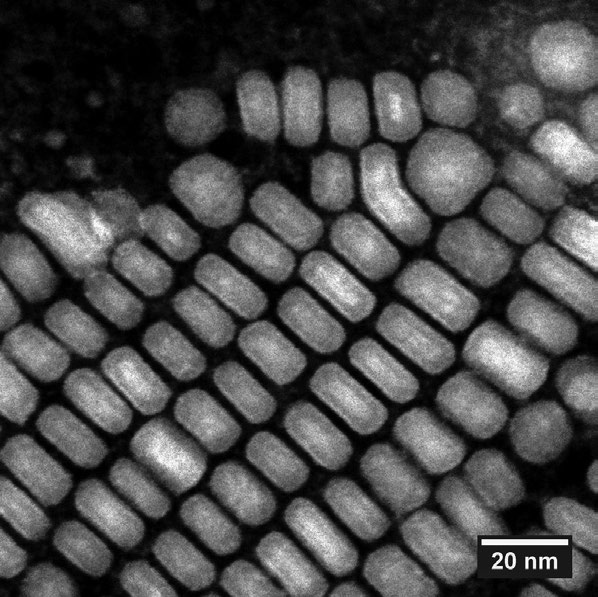
Figure 2. SEM image of nanoparticles with scale. Image Credit: Entegris

Figure 3. Fluorescent nanoparticles (quantum dots). Image Credit: Entegris
Light Scattering Techniques
A number of light scattering methods may also be used to measure particle size. These include:
- Small Angle Neutron Scattering (SANS)
- Laser diffraction
- Small angle X-ray
- Dynamic Light Scattering (DLS)
Dynamic Light Scattering (DLS) is the most commonly used light scattering technique. DLS’ basic principle involves the time signature of the scattering triggered by the Brownian motion of the particles - smaller particles will diffuse more quickly than larger particles.
Figure 4 displays a simplified optical diagram of a typical DLS system.
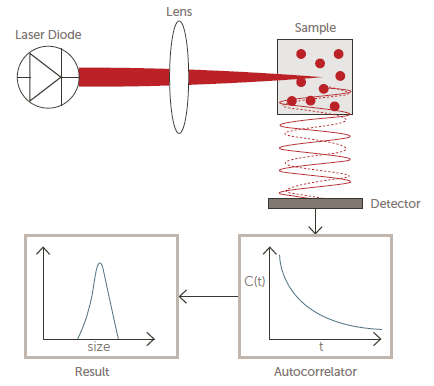
Figure 4. Optical diagram of a DLS system. Image Credit: Entegris
Spectroscopic Techniques
It is possible to use the interaction between particles and electromagnetic radiation as a function of wavelength to determine the size and other properties of certain classes of nanoparticles. Spectroscopic techniques such as these may include:
- Nuclear Magnetic Resonance (NMR)
- Fluorescence (Figure 3)
- UV – Visible
- Infrared (IR)
A detector will count photons before feeding this raw data into a correlator. The measured correlation function is then used to ascertain the diffusion coefficient (D), which is then used to calculate particle size using the well-known Stokes-Einstein equation:
D = kT/6π ŋR
Where:
- D = Diffusion coefficient
- R = Particle radius
- k = Boltzmann’s constant
- T = Temperature Kelvin
- ŋ= Shear viscosity of the solvent
Other Techniques
A number of other particle characterization techniques are often used in the investigation of nanoparticles. These include:
- BET specific surface area (SSA) - used to calculate mean particle size
- Mass Spectrometry (MS) – used to determine particle mass
- X-ray diffraction – used to ascertain crystal structure
- Electrophoretic Light Scattering (ELS) – used to determine particle charge
Particle charge (zeta potential) affects nanoparticle stability and is a function of the specific surface chemistry of a dispersion. Zeta potential is measured via Electrophoretic Light Scattering (ELS).
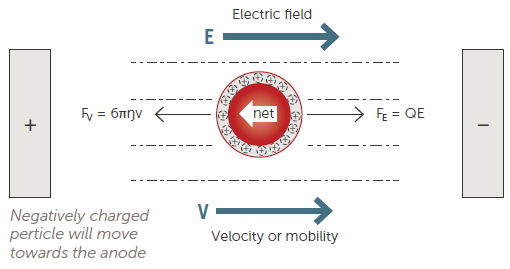
Figure 5. Electrophoretic Light Scattering (ELS) technique. Image Credit: Entegris
This is commonly done by applying an electric field to the sample before measuring the speed and direction of the particle motion (Figure 5).
The direction of the particles will indicate if they are positively or negatively charged, while their speed will indicate the magnitude of the charge. It is possible to measure particle velocity using either phase analysis light scattering (PALS) or frequency analysis.
PALS is a newer and more sensitive approach, leading to this now being the preferred approach for working with the majority of samples.
Examples and Applications of Nanoparticles
Nanoparticles have become commonplace in a range of products, primarily due to their unique properties and advantages over traditional materials.
A number of examples of incorporating nanoparticles are provided below, and in many instances, these applications will result in a new product that has improved and more desirable properties.
Table 1. Source: Entegris
| . |
. |
Automobile
tires |
To improve mechanical properties and wear |
| Polymers |
Nanoclays added to polymers improve strength and impact resistance |
Food
packaging |
Nanoflakes of clay moderate moisture and gas through the film |
Paint and
coatings |
Antibacterial properties for hospitals and medical facilities |
Flame
retardants |
Used in plastics, replace flammable organic halogens for lower emissions |
| Batteries |
High surface area of the nanoparticles increases storage capability |
| Ceramics |
The nanoparticles with polymers create more resiliency and can add electrical properties |
Diode white
light |
Coating the bulb to modify the wavelengths can create a white light LED |
| Sunscreen |
Optisol replaces traditional sunscreen ingredients, eliminating the health risk |
| Medicine |
Small sizes can circulate throughout the body delivering payloads of drugs to specific areas, cells, tumors and other diseased tissue. Can be used to enhance images on MRI and PET. Drug delivery to the brain via inhalation has considerable promise for Parkinson’s, Alzheimer’s, and Multiple Sclerosis. |
Nanoparticles for Drug Delivery
One novel and potentially ground-breaking area where nanoparticles are being employed are in drug delivery. A significant number of nanoparticle-based drug products are being developed to enhance targeting.
A passive targeting approach has the potential to increase circulation time by reducing the nanoparticle’s size and cloaking it with a coating such as polyethylene glycol (PEG).
An active targeting approach will modify the nanoparticle’s surface, allowing this to better seek and adhere to specific parts of the body, for example, adhering to cancer tumors whilst avoiding healthy tissue.
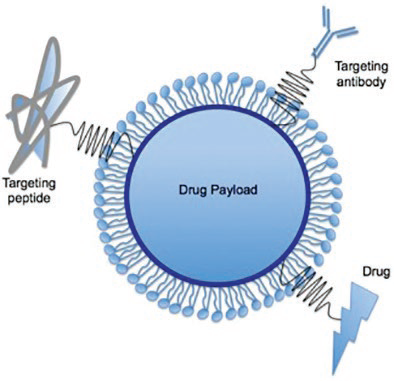
Figure 6. Surfaced modified nanoparticle for drug targeting and delivery.13 Image Credit: Entegris
Cell-specific ligands on the nanoparticle’s surface can be added in order to bind specifically to complementary receptors (Figure 6).
It is essential that the size of nanoparticles used for drug delivery is properly controlled. This necessitates the use of proper analytical methods to ensure that the size is within the required specifications. DLS remains the most frequently utilized particle sizing technique for these types of applications.
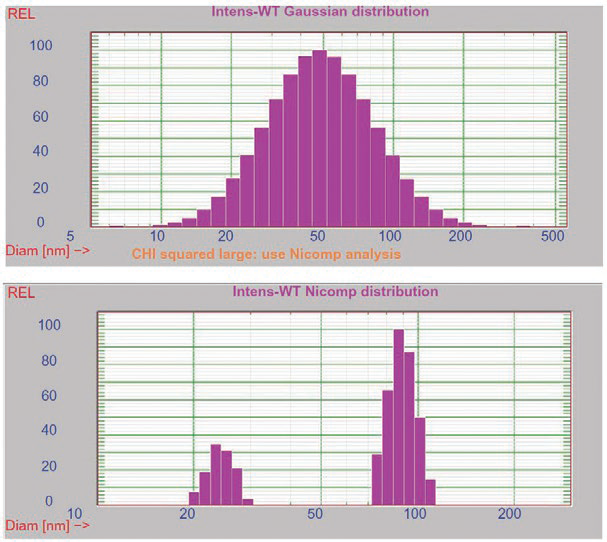
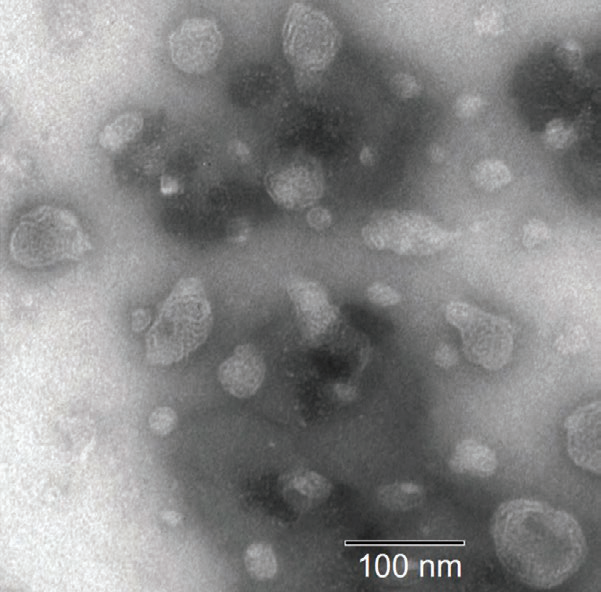
Figure 7. DLS and SEM results for liquid crystalline nanoparticles.14 Image Credit: Entegris
Figure 7 displays DLS and SEM results14 for a series of lipid-based liquid crystalline nanoparticles (LCNPs). These self-assembled structures are prepared through the high shear energy dispersing of a nonlamellar liquid crystalline matrix into its water phase.
In this example, both SEM and multimodal DLS (lower left graph) confirm the presence of larger (90 nm) and smaller (25 nm) size populations.
Conclusions
Creating, controlling and effectively utilizing nanoparticles is an essential part of the wider nanotechnology field. Considerable research and development into nanoparticles for a range of uses are taking place in both industrial and academic laboratories.
Nanoparticle-based products are already available in a number of industries, while nanoparticles for drug delivery offer the potential for significant health benefits.
References
- Lee, J., Curr Nanosci, Volume 1, Number 3, 2005: pp. 263–266
- National Nanotechnology Initiative Budget, available at www.nano.gov
- The Maturing Nanotechnology Market: Products and Applications, NAN031G, Nov 2016, bbc Research
- Global Nanotechnology Market- Industry Trends and Forecast to 2025, Data Bridge Market Research
- ASTM E2456-06(2012), Standard Terminology Relating to Nanotechnology, www.astm.org
- ISO/TS 27687:2008, Nanotechnologies -- Terminology and definitions for nano-objects -- Nanoparticle, nanofiber and nanoplate, www.iso.org
- ISO/TS 80004-4:2011, Nanotechnologies – Vocabulary -- Part 4: Nanostructured materials
- ISO/TS 80004-4:2011, Nanotechnologies – Vocabulary -- Part 5: Nano/bio interface
- ISO/TS 80004-4:2011, Nanotechnologies – Vocabulary -- Part 7: Diagnostics and therapeutics for healthcare
- National Nanotechnology Initiative Strategic Plan, February 2014; available online at https://www.nano.gov/sites/default/files/pub_resource/2014_nni_strategic_plan.pdf
- Scientific basis for the definition of the term “nanomaterial”, March 2012, DOI: 10.2772/39703, Publisher: Ed. Publications Office of the European Union, Luxembourg, Luxembourg, 46 pages (2012)
- Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, 2014, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology
- Graphic source: https://en.wikipedia.org/wiki/Nanoparticles_for_ drug_delivery_to_the_brain Graphic by Andrea Trementozzi – Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=30082797
- Zeng et al., Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption, International Journal of Nanomedicine 2012:7

This information has been sourced, reviewed and adapted from materials provided by Entegris.
For more information on this source, please visit Entegris.