October 1st is International Coffee Day, but coffee is a drink we enjoy all year round. But, there are a number of factors that determine what makes a good quality cup of coffee, from how the beans are processed to the origin of the beans and the climate they grow in, to how they are roasted and packaged, and how the roasted beans are ground and brewed.

Image Credit: Metrohm Middle East FZC
This article will outline a short history of coffee, how it is processed and how to accurately establish the quality parameters to brew the most flavorful cup of coffee.

Image Credit: Metrohm Middle East FZC
Origins of Our Favorite Brew
The word coffee is derived from the Dutch ‘koffie’ and was introduced in 1582. This traces back even further to the Arabic word for coffee, ‘qahwa’, which has been speculated to come from ‘quwwa’ (defined as power or energy), or even from Kaffa (also spelled as Kefa), a medieval Ethiopian kingdom which exported coffee plants to Arabia.
It is thought that it was a goat herder in Ethiopia that first discovered coffee, who noticed that after consuming the coffee fruit (known as cherries), the energy of his goats increased. During the 15th and 16th centuries, coffee consumption spread through the Middle East and Arabian Peninsula from Ethiopia.

Coffee cherries ripening. Image Credit: Metrohm Middle East FZC
Except for water, coffee is now the most consumed beverage around the world, from transparent drip coffee to the thickest espressos. This beverage is so deeply ingrained in all cultures that contestants from South Korea, Greece and Canada have won the top places for the annual World Barista Championships in 2019.
Good Coffee: Not as Straightforward as You Might Think
Coffee is available in various forms, with niche roasters looking to discover new flavors daily. Specialty coffee is also in high demand as the gourmet coffee market is growing. In 2020, Global Brands Magazine reported the price of Black Ivory Coffee at $ 500 per pound.

Civet cats can digest coffee beans to create a unique coffee experience with a heavy price tag. Image Credit: Metrohm Middle East FZC
Why is it so Expensive, is the Taste that Good?
The coffee cherries are fed to and digested by elephants to make Black Ivory Coffee. This is a similar process to Kopi Luwak (or civet coffee), which is another expensive coffee variety created by the fermentation of coffee cherries in the gut of civet cats. The resulting coffee beans are then cleaned, dried and roasted.
Other varieties of coffee beans are roasted in large amounts for mainstream consumption (robusta, arabica and liberica) aside from these high-priced small batches. The global market is made up of 60% Arabica beans, with 2.5 million tons per year exported from Brazil alone.
Robusta beans are mostly produced in Vietnam and account for just below 40% of the market. Robusta beans contain more caffeine, have a more bitter flavor and are utilized to create instant coffee.
Compared to the other two major species, Liberica beans have low concentrations of caffeine but high levels of sugars. This type is more difficult to mass-produce due to very low yields (between two and four times lower than the others) and larger plant size, so it only makes up around 2% of the global coffee market.
A number of varieties have been produced with a wide range of different flavor characteristics and caffeine content using these major coffee species. The ideal climate differs depending on the species, but usually, coffee is grown in (sub)tropical areas.
Some are more suitable for mountainous regions as they prefer higher altitudes. Others need dry, hot conditions to produce the best quality beans.
There are now over 70 countries that produce coffee, which is good because global coffee consumption in 2020/2021 is estimated to be 167.23 million 60 kg bags – that is over 10 million tons of coffee.
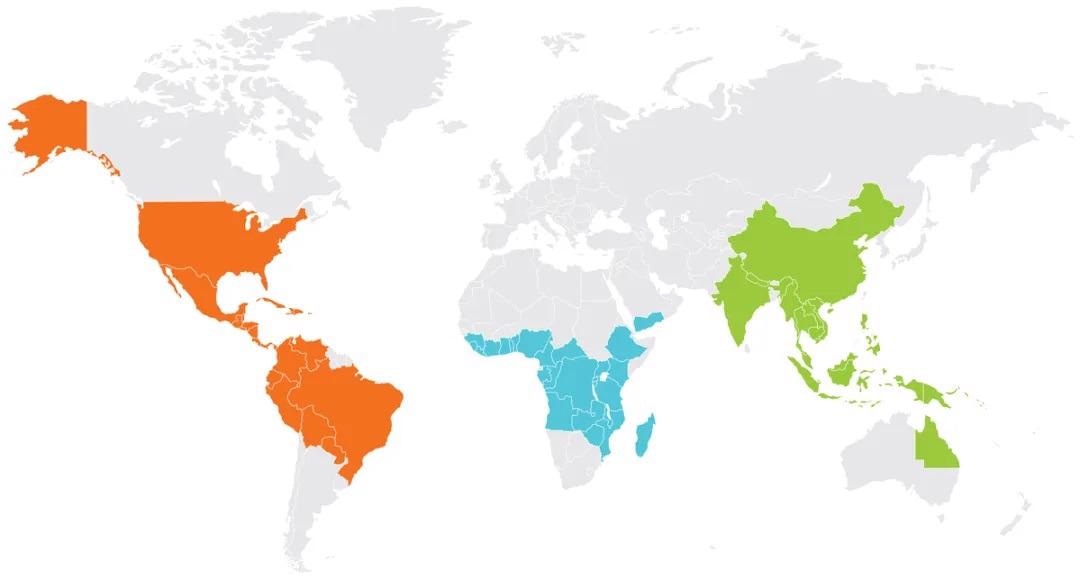
Map showing the different coffee-producing countries around the world. Image Credit: Metrohm Middle East FZC
Changes in Coffee Consumption Practices
Over the past decade, the adoption of the pod coffee machine (e.g., Nespresso, Keurig) has pushed the consumption of coffee to a much higher rate of consumption at home, from something typically enjoyed in a café, restaurant or on the go.
With this significant shift to pod coffee, the ability to adjust water temperature, grind size or extraction time utilized by the best baristas to counter alterations in strength and flavor is no longer a possibility.
In fact, pod coffee is so popular due to the ease of pressing a single button and receiving hot, fresh coffee within seconds. This puts new pressures on coffee roasters to maintain the caffeine strength and flavor expected of their brand and varieties.

Image Credit: Metrohm Middle East FZC
Though a number of people think that the coffee brewing process is the largest contribution to a good cup of coffee, numerous other quality parameters, including roast temperature, acidity and water quality, contribute even more.
Two of the main factors in an optimal cup of coffee, the amount of caffeine and the acidity (taste), are mainly affected by the region of origin, bean type and roasting temperature.

Progression of the coffee bean roasting process. Image Credit: Metrohm Middle East FZC
Science — Brewing up Your Perfect Cup
Science enables us to define a number of the key quality parameters which lead to the caffeine strength and taste we expect from our favorite brand of coffee. Coffee has a pH of around five and is generally acidic. Highly acidic coffee exhibits a harsh, sour flavor.
While on the consumer side, there are ways to counteract this, for manufacturers, it is even more vital to identify that there is an issue to start with. The titratable acidity of the coffee is a simple identifier, and this has a direct correlation with the taste you associate with your favorite brew.

Image Credit: Metrohm Middle East FZC
The ‘kick’ you may get from your preferred caffeine fix is equally important. The recommended daily limit for adults is suggested to be 400 mg caffeine, whether you drink one cup per day or four. Of course, decaffeinated coffee is also a choice for those who are sensitive to its effects or are looking for ways to decrease their intake.
After a long sample preparation procedure, caffeine has traditionally been analyzed by titration, liquid chromatography (LC) or spectrophotometry. Now, the analysis of key coffee quality parameters, like caffeine content, can be performed simply and effectively by utilizing a single titration system.
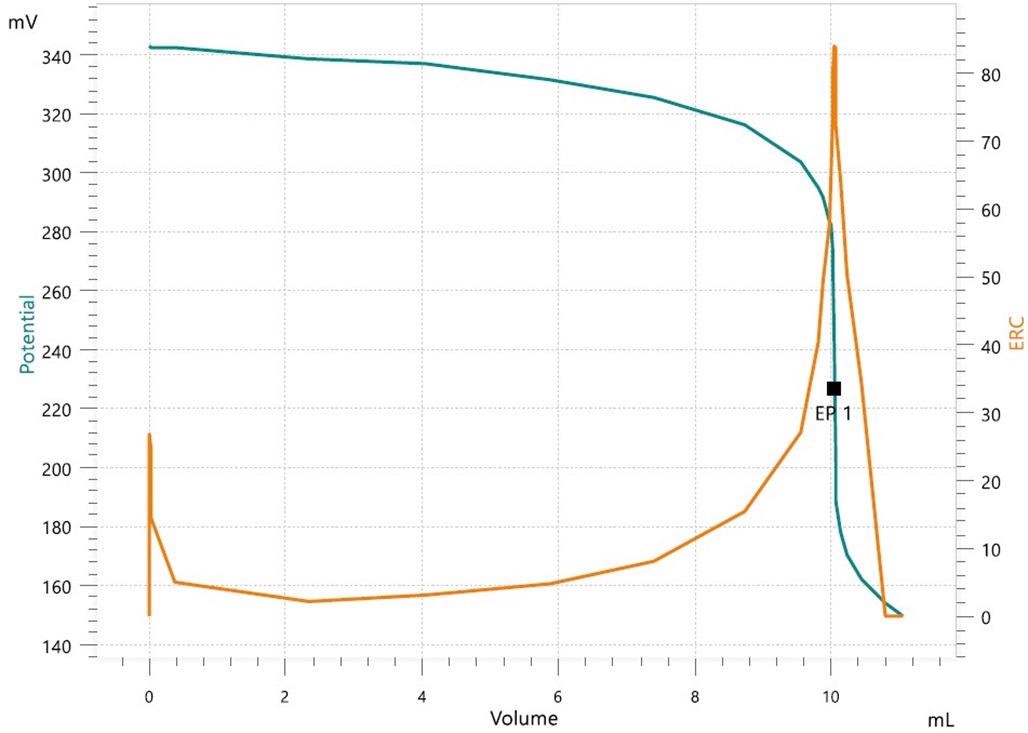
Example titration curve for caffeine analysis with OMNIS. Image Credit: Metrohm Middle East FZC
The acidity and pH of coffee samples are analyzed by utilizing a robust pH electrode during titration against standardized sodium hydroxide.
After a known excess of iodine is added to the sample and left to react, caffeine is established via a redox back titration. The sample is filtered and titrated with sodium thiosulfate after the reaction period.
Metrohm has the Solution for Your Analysis Needs
Thanks to minimal manual sample preparation steps, the OMNIS platform from Metrohm supplies laboratory analysts with the automation they require to make each sample determination much faster, simpler and more reproducible.
Key steps in the analysis process which have previously needed manual interactions, reagent addition, filtration and accurate volume transfers are now completed accurately and automatically.

Image Credit: Metrohm Middle East FZC
Metrohm Middle East FZC (MME) based out of Sharjah, UAE is the Regional Support Centre for Metrohm AG (Switzerland) which is responsible for sales, service and calibration of lab & process analytical instruments from the following countries –
UAE, KSA, Kuwait, Bahrain, Oman, Qatar, Egypt, Jordan, Lebanon, Iraq, Bangladesh, Pakistan, Sri Lanka, Ethiopia, Ghana, Sudan, Syria, Yemen, Somalia, Iran, Cyprus, Malta, Eritrea, Djibouti & Afghanistan.
Acknowledgments
Produced from materials originally authored by Isaac Rogers and Dr. Alyson Lanciki from Metrohm International.

This information has been sourced, reviewed and adapted from materials provided by Metrohm Middle East FZC.
For more information on this source, please visit Metrohm Middle East FZC.