Proximate analysis is a long-serving, widely used approach for determining the rank of coals. This method works by separating out inert components, volatile components and fixed carbon.
The quality of coal products is extremely wide-ranging, each possessing a distinct commercial value. There is a clear need to rank these products and, in turn, a clear need for robust methods to achieve this.
A number of distinct ASTM® tests can be used to perform these separations via highly specialized industrial equipment.1 These methods see tests performed with gram-sized samples, an approach designed to make obtaining a representative, smaller sample much easier.
Round robin testing is frequently conducted using homogenized sample materials and multiple laboratories, which are then identified and documented. This method has led to the development of a number of approaches to reliably perform this coal-ranking separation.
The wide range of volatile and pyrolytic components present in coal samples means that this approach incorporates an empirical separation involving an arbitrary aspect. Standard samples are typically used to enable testers to finetune their testing conditions to standardize their analysis as much as possible.2
Standard samples are provided in a -60 mesh (250-micron diameter particles), fine enough to facilitate the testing of samples in the 10 to 100-milligram size range while removing the risk of significant statistical sampling error.3,4
PerkinElmer was one of the first developers of a methodology for proximate testing by TGA of coke and coal.5,6,7,8
The company now has over 30 years of experience in this technique, applying its experience and expertise to a diverse array of carbonaceous products.9 Figure 1 displays a TGA protocol proximate analysis of coal using the STA 8000 instrument.
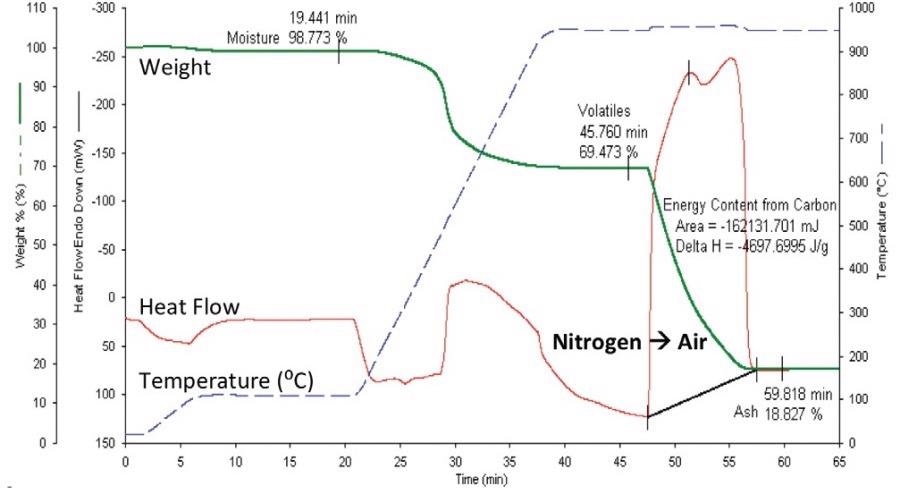
Figure 1. TGA Proximate analysis of coal using the STA 8000. Image Credit: PerkinElmer
Instrument Requirements
Proximate testing by TGA has modest analytical requirements. Users must be able to accurately record the weight of a sample as this is heated over a specified temperature range and held isothermally at key temperatures.
Users must also have the capacity to alter the sample’s environmental atmosphere from inert to oxidizing.
The PerkinElmer STA 8000 is able to confidently accommodate these requirements, featuring accurate temperature and gas control capabilities, a balance with microgram sensitivity and easy-to-use, powerful software designed to enable and automate analysis.
The instrument’s top loading balance also offers a number of key advantages for this analysis.10
The study presented here aims to illustrate the proximate analysis of two standard samples using the STA instrument, highlighting its distinct combination of performance, moderate cost, small profile, robustness and excellent applicability for this type of routine analysis.
The STA 8000
PerkinElmer provides a number of analyzers that are able to perform this analysis. Both the Pyris 1 TGA and the TGA 4000 can accommodate autosampler accessories, making them ideal choices for facilities looking to handle significant sample throughput.
The STA 8000 analyzer (Figure 2) boasts a range of beneficial features that make it well suited to this application.

Figure 2. The STA 8000 Simultaneous Thermal Analyzer10. Image Credit: PerkinElmer
The instrument’s weighing mechanism – a top loading balance – is ideally suited to routine quality testing applications. This makes it easier to load than the taut-band fulcrum setup, and no force is applied to the hang-down wire or a long balance arm during loading that could damage the balance system.
Unlike horizontal TGAs and STAs, sample position does not impact the movement arm between the sample and the fulcrum. This arm determines the weighing constant, meaning that there is no evident weight change if the sample shifts on melting.
Should the sample be loaded into the pan asymmetrically, this will not impact the apparent weight. STA capsules are also better equipped to keep the ash in place compared to a boat-shaped sample holder or a system whereby purge flows across the sample directly.
The instrument’s compact, low mass furnace is closely coupled to the sample, ensuring that the vital atmosphere switchover from inert to oxidizing is quick and comprehensive.
The study employed a range of purge gas flow rates from 40 to 200 cc/minute, and the positioning of the balance below the furnace, separated by a purged channel, ensured that any pyrolysis products did not contaminate the balance.
Experimental
Two standard sample materials were obtained from Alpha Resources:
- a metallurgical coke standard (AR-733)
- a bituminous coal standard (AR-1720)
Both samples were initially run using a TGA proximate analysis technique (Figure 1).
This temperature program involved sample loading and weighing at 25 ˚C, followed by heating to 110 ˚C, holding for a total of 10 minutes before heating to 950 ˚C where the temperature was then held until a constant weight was achieved in nitrogen.
Next, the sample atmosphere was switched to air after being somewhat diluted by the balance purge.
Once the weight was constant, following combustion of the fixed carbon component, the analysis process was halted, and the apparatus was allowed to cool prior to the next analysis. This yielded consistent values, but these values were found to be different from those certified.
The most notable difference is that the certified sample tests such as ASTM® tests will presume that samples have been thoroughly dried to a constant weight at 107 ˚C before testing commences. Figure 3 highlights the significance of this post-drying proximate test.
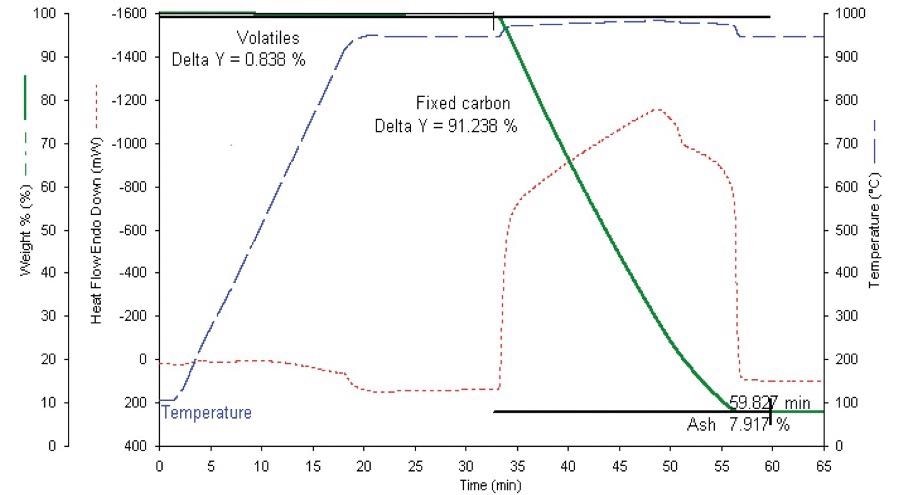
Figure 3. Proximate analysis of metallurgical coke. Image Credit: PerkinElmer
Results and Discussion
Total Volatiles
For the determination of the total volatiles, the sample is heated from 107 ˚C to 950 ˚C before being held isothermally to separate out any volatile components present. Figure 4 displays this initial analysis step for the metallurgical coke sample on an expanded scale.
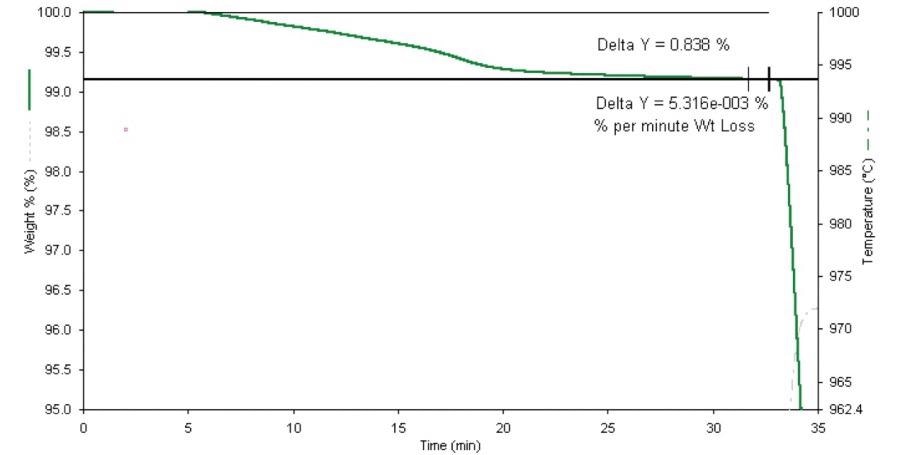
Figure 4. Proximate analysis of coke (expanded scale). Image Credit: PerkinElmer
After a 20-minute period, weight loss at this temperature was generally found to be less than 2 µg per minute, effectively demonstrating that no notable amounts of oxygen are entering the STA.
Other tests showed that a purge rate of 40 cc per minute was enough to stop any measurable back diffusion of oxygen from air into the sample and/or furnace chamber.
A purge rate of 100 cc per minute was used to acquire the data presented below. The switch-over to air or oxygen required for this combustion step can be set to automatically take place at a specific time or when a particular rate-of-weight-loss criterion is met.
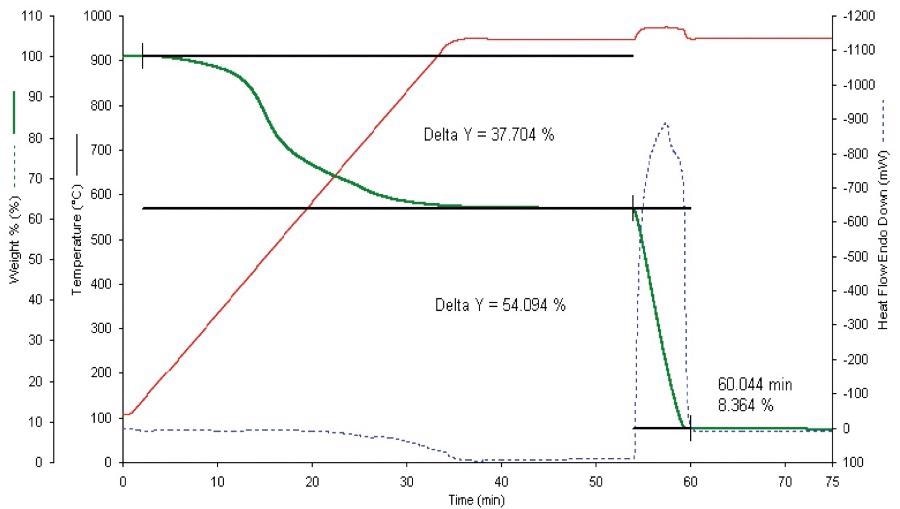
Figure 5. Proximate analysis of AR-1720 Std. Coal. Image Credit: PerkinElmer
Agreement with Certificate
Results for the AR-733 coke sample were all found to be within the accepted degree of uncertainty of the certificate values (Table 1), even despite the range of sample sizes investigated.
Results for the AR-1720 coal sample revealed ash results that fell within 0.1% of the certificate values (Table 2).
Results for the volatiles saw the percent found returned low by 1.4%, and high by 1.7% for the fixed carbon.
These results were expected to some degree because the ASTM® method calls for a specific sample geometry which is prone to exposing a far higher surface area for volatiles off-gassing.
Table 1. Results for metallurgical coke standard AR-733. Source: PerkinElmer
| Sample Wt (mg) |
Volatiles % |
Carbon % |
Ash % |
| 30.82 |
1.03 |
91.01 |
7.81 |
| 70.25 |
0.84 |
91.24 |
7.92 |
| 107.8 |
0.46 |
91.05 |
8.03 |
| 89.96 |
0.81 |
91.02 |
8.12 |
| Average |
0.785 |
91.08 |
7.97 |
| Average Deviation |
0.162 |
0.080 |
0.105 |
| Certificate value |
0.85±0.36 |
91.11±0.36 |
8.04±0.42 |
Table 2. Results for coal standard AR-1720. Source: PerkinElmer
| Sample Wt (mg) |
Volatiles % |
Carbon % |
Ash % |
| 25.04 |
37.7 |
54.09 |
8.36 |
| 22.46 |
37.01 |
54.09 |
8.99 |
| 61.04 |
37.98 |
54.51 |
7.68 |
| 86.49 |
37.75 |
54.22 |
7.73 |
| Average |
37.61 |
54.2275 |
8.19 |
| Average Deviation |
0.30 |
0.14 |
0.48 |
| Certificate value |
39.17±0.45 |
52.54 |
8.29±0.07 |
Calorific Calculation
The STA 8000 simultaneous thermal analyzer offers users the ability to directly determine energy linked to thermal events, for example, the combustion of carbon.
This simple calculation can be performed on the proximate analysis’ carbon oxidation step, but a more accurate assessment of the calorific content of coke or coal can be calculated using the fixed carbon and coal rank approaches.8
Conclusion
The data highlighted in this study showcases the STA 8000’s ability to provide reproducible proximate analysis of coal and coke.
As this is an empirical analysis, dwell times and separation temperatures could require adjustment to acquire appropriately identical results to those acquired via commercially accepted large sample ASTM® tests.
This study is just one of several that illustrate the usefulness of proximate analysis performed using a TGA or STA analyzer for users looking to rapidly analyze small quantities of coke and coal.
References
- See ASTM® Methods: ASTM® D 3302-12, ASTM® D 3175-11, ASTM® D 3174-11, and ASTM® D 7582-10e1.
- Alpha Resources, Stevensville, MI.
- Culmo, R.F., Swanson, K., PerkinElmer Publication EAN-26.
- “Guidance for Obtaining Representative Laboratory Analytical Subsamples from Particulate Laboratory Samples” https://www.epa.gov/.
- Earnest, C.M., “Thermal Analysis in the Coal Industry,” Proc. of the 4th International Coal Testing Conf., Lexington, Ky, Oct. 1984, pp219-232.
- Earnest, C.M., and Culmo, R.F., “The use of Modern Computerized Instrumentation for the rapid Proximate and Ultimate Analysis of Coals,” Proc. of the 3rd International Coal Testing Conference, Lexington, KY, Oct.1983, pp66-70.
- Earnest, C.M., ”A Thermogravimetric Method for the Rapid Proximate and Calorific Analysis of coal and Coal Products,” Thermal Analysis Vol. II; Proc of the 7th ICTA, Miller, B., Ed., 1982, pp 1260-1269.
- Earnest, C. and Fyans, R.L., “Recent Advances in Microcomputer Controlled Thermogravimetry of Coal and Coal Products”, Thermal Analysis Application Study #32.
- Elder, John., Thermogravimetry of Bituminous Coal and Oil Shales”, Proc. of the 10th NATAS Conf., October 1980.
- STA 8000 brochure, PerkinElmer publication 010452_01.
Acknowledgments
Produced from materials originally authored by Bruce Cassel and Kevin Menard from PerkinElmer, Inc. and Professor Charles Earnest from the Dept. of Chemistry at Berry College, Mt. Berry, GA USA.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.