The ever-present hydrogen economy has gained renewed momentum in the energy transition as leaders in both the public and private sectors push to decarbonize the industrial economy.
Hydrogen, a clean-burning molecule, can be generated without emissions. This can be achieved through traditional methods like Steam Methane Reformation (SMR), gasification processes with carbon capture, or electrolysis, which leverages low-cost or surplus renewable energy from the electric grid.
For challenging thermal loads that are difficult to electrify, such as “greening” refineries and chemical processes, hydrogen emerges as the optimal choice for achieving a net-zero economy.
Image Credit: Endress+Hauser
There is an ongoing and robust debate regarding the prioritization of hydrogen utilization, given its comparatively high production costs compared to traditional and alternative fuels and energy carriers. However, in the context of a carbon-neutral society, its adoption is inevitable.
By 2050, hydrogen production is expected to make up 12% of the world’s energy supply with a wide range of novel applications like blending hydrogen in Natural Gas or Utility Scale Electrolyzers (IRENA).
Endress+Hauser, a leading manufacturer of process instrumentation, plays a pivotal role across the entire value chain of hydrogen, spanning from production to end use.
With a rich history of providing guidance and participating in hydrogen processes, Endress+Hauser offers specialized expertise to address the evolving challenges encountered in this resurging market. One crucial aspect of measurement, pressure, faces a specific risk: the permeation of hydrogen through the process membrane.
What is Hydrogen Permeation?
In direct hydrogen gas service, the diaphragm surface of the pressure transmitter experiences the dissociation of hydrogen molecules.
Next, hydrogen atoms undergo electron loss, allowing the diffusion of H+ ions through standard 316 L or Alloy C diaphragms.
Upon reaching the opposite side of the metal diaphragm, the H+ ions regain electrons and recombine into H2 molecules. These newly formed H2 molecules then dissolve in the fill fluid of the pressure cells.
The rate and extent of permeation are influenced by the process pressure and temperature.
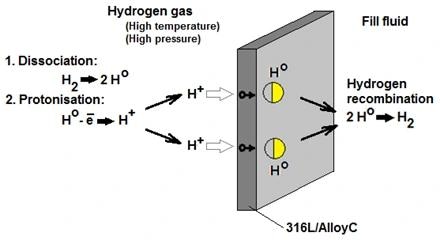
Image Credit: Endress+Hauser
Why is Hydrogen Permeation Detrimental to Pressure Transmitters?
The risk associated with hydrogen permeation is not immediate. As long as an adequate process pressure is maintained on the diaphragm, the H2 molecules will remain dissolved. Only when the process pressure drops to near or below atmospheric pressure does damage occur.
When the process pressure decreases, H2 molecules within the fill fluid emerge from the solution and form gas bubbles. This phenomenon occurs rapidly, leading to an increase in fluid volume and internal pressure.
Due to the closed environment of the pressure cell or diaphragm seal, this additional volume lacks an outlet for escape. Consequently, the thin 316 L/Alloy C membrane becomes distended, resulting in catastrophic damage.

Image Credit: Endress+Hauser

Image Credit: Endress+Hauser
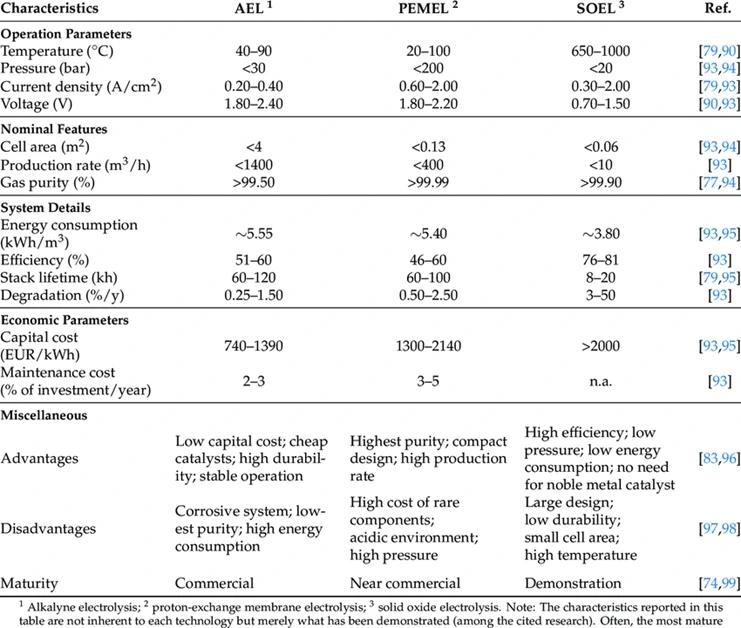
Hydrogen Production
Three emerging technologies exist for large-scale electrolysis, which involves using high DC currents to split H2O into its constituent elements, H2 and O2.
The importance of pressure is significant, as many of these technologies rely on differential pressures across the membrane.
Once the H2O is converted, the resulting hydrogen gas is dried and treated for its intended end use. Monitoring outlet pressure and differential pressures across critical components becomes crucial for assessing process health and, in certain cases, complying with Safety Instrumented Systems (SIS) or functional safety requirements.
Consequently, the risk of hydrogen permeation through pressure transmitter diaphragms is a constant concern due to the diverse process conditions associated with these novel production techniques, as they directly affect the permeation risk of hydrogen.
Source: MPDI. Image Credit: Endress+Hauser
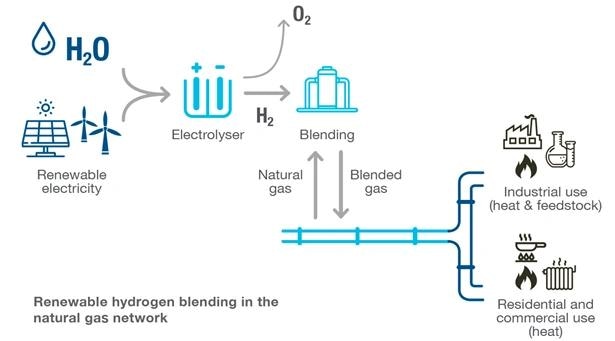
Hydrogen Blending
There are more complicated matters at hand, deviating from the typical hydrogen applications of the past.
Recent power plant demonstrations, exemplified by Long Ridge Energy’s Hydrogen Ready Power Plant in Hannibal, Ohio, have revealed a growing interest in the integration of hydrogen into natural gas. This integration aims to reduce the carbon content in gas pipelines and fuel systems.
The accompanying image illustrates the application of this process in pipelines, where compressors are employed to inject the blended gas back into the pipeline. Maintaining a higher injection pressure than the pipeline pressure is crucial to ensure the successful introduction of the blended gas into the pipeline.
The image below represents the pipeline application.
Source: Australian Energy Council. Image Credit: Endress+Hauser
Compressors are used to inject blended gas back into the pipeline. It is critical to keep the injection pressure higher than the pipeline pressure to ensure the blended gas will be pushed into the pipeline.
Any pressure transmitters on the blended gas line and downstream of this injection site would be subject to some increased percentage of hydrogen by volume. Failures in pressure measurements could cause a potential trip in the control loop if the supply pressure faults.
The risks of permeation vary across different blends of hydrogen, which adds complexity for end users and engineering firms involved in designing new systems or assessing the capabilities of existing infrastructure concerning the potential percentage of hydrogen by volume.
What Can Be Done to Mitigate the Risk of Hydrogen Permeation?
The common approach involves applying a gold (Au) coating to the outer surface of the diaphragm.
This coating increases the density of the diaphragm, creating a diffusion barrier that hinders hydrogen permeation. When properly used, the gold coating can reduce hydrogen permeation by up to 106 times due to its extremely low diffusion coefficient.
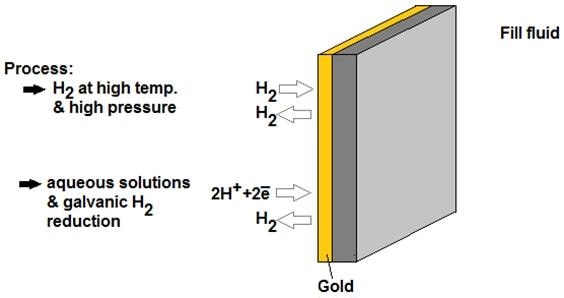
Image Credit: Endress+Hauser
What Thickness of Gold Coating Should You Specify for Your Hydrogen Applications?
While there is a wide range of coatings available, not all of them are suitable for preventing hydrogen permeation. A coating thickness of 25 µm is typically recommended as it strikes a good balance between protection and cost.
Thinner coatings are more challenging to apply evenly and carry the risk of developing pinholes, which would reduce the level of protection. Coatings thicker than 25 µm are also available, but they come with increased cost and lead time.
Hydrogen permeation is persistent when it comes to metal diaphragms. No coating can completely prevent it from occurring. However, when appropriately applied, a gold coating provides a cost-effective method to significantly reduce hydrogen permeation and prolong the lifespan of the pressure transmitter.
What if Hydrogen is Blended with Natural Gas?
Although reducing the volume of hydrogen present will also decrease the risk of hydrogen permeation, it does not eliminate it. For a conservative design approach, even in blended applications, it is advisable to use gold-coated membranes.
If hydrogen blending is temporary or constitutes less than 10% of the volume, traditional 316 L stainless steel membranes may be considered.
References and Further Reading
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. https://doi.org/10.3390/app112311363

This information has been sourced, reviewed and adapted from materials provided by Endress+Hauser Ltd.
For more information on this source, please visit Endress+Hauser Ltd.