Introduction
The study of semiconductor nanoparticles embedded in several types of matrix is currently a very active research area. Small particles, with size in the range of 1-20 nm, have physicochemical properties quite different from those observed in the bulk material [1]. A great variety of semiconductors nanoparticles have been synthesized in different matrix such as polymers, glasses and zeolites with the main purpose to modify their properties by controlling the particle size. Thus, the interest on these systems has been driven because the possibility to tailor the properties of a material for specific technological applications in the fields of non-linear optics, photovoltaic conversion, catalysis, optoelectronics, etc. Zeolites are nanoporous crystalline materials composed by Si, Al and O atoms, which constitute a framework structure of cages interconnected through channels with molecular dimensions. There are different techniques to incorporate compound semiconductors into the well defined and ordered zeolite cages. The cages and channels of the zeolite matrix, both of nanometric dimensions, are very appropriate sites to self-assemble and stabilize semiconductor clusters. Several semiconductor-zeolite systems have been studied and reported in literature [2-6].
The modifications of the properties of a semiconductor material are much stronger when its dimensions are comparable to the exciton Bohr diameter. PbS is a IV-VI compound semiconductor with narrow energy band gap, 0.41 eV at 300 K, high dielectric constant, 17.3 and small electron effective mass, <0.1 m* [7]. The values of the last two parameters yield to a large exciton Bohr diameter, about 18 nm [8-10], for this semiconductor material. Therefore, PbS is an adequate material to study particle size effects because it is not very difficult to synthesize PbS nanoparticles smaller than 18 nm. In fact, the strong confinement regime in which the particle size is much less than the exciton Bohr radius, has been achieved in systems with PbS nanoparticles having a size between 1-4 nm [11-15]. In this work we have synthesized PbS nanoparticles with an average size of 4 nm in zeolite Na-X, by means of ionic exchange processes in alkaline aqueous solutions. We report here the optical and structural properties of the system PbS-Na-X zeolite.
Experimental
The zeolite used for the preparation of our samples was a synthetic zeolite X from Waco Chemicals Inc. with formula Na2OAl2O3 2.5SiO2 . This zeolite is of the faujasite type with a silicon-aluminum ratio of 1.25 and a porous size of 0.9 nm. The method to synthesize the PbS inside the zeolite matrix is based in the alkaline conditions of the reaction solution and in the employment of thiourea, CS(NH2)2, as the sulfide source in the alkaline solution. The synthesis process was done as follows: The zeolite Na+-X was first treated with a solution 0.1M of NH4NO3 for 5 hours at reflux temperature (about 100 ºC). Then, the mixture was separated by filtration and the product was successively washed: once with deionized water, 6 times with a solution 0.06M of HCl, 6 times with a solution of 0.06M of NaOH and 7 times with deionized water. After the washing process the treated zeolite was dried at room temperature. The intention of this step is the interchange of Na+ ions in the zeolite cavities by NH4+ ions, which decomposes as NH3 (gas) + H+. Thus, after this step we obtained zeolite Na+H+-X. The Pb2+ ion exchanging process in the zeolite was done as follows: One gram of zeolite Na+H+-X was added to a solution of 20 ml of 0.1M lead acetate. This mixture was magnetically stirred at a temperature of 50°C for 2 hours. The Pb2+-exchanged zeolite was filtered and extensively washed with deionized water until no Pb2+ ions were found in the filtered liquid by dropping Na2S. To make the reaction with the S2- ion, one g of Pb2+-exchanged zeolite was added to a solution of 40 ml of 0.1M thiourea and magnetically stirred by two hours. Three samples were prepared by making this sulphidation process at the temperatures of 55, 65 and 75°C, respectively. Finally, the samples were washed with deionized water and collected by filtration. The obtained samples were fine powders with lackluster white color. The three samples were stable at ambient conditions, that is their color did not change when exposed to the contact of atmospheric moisture. The samples were studied by x-ray diffraction (XRD) measurement using a Rigaku D/max-2100 diffractometer. The optical absorption spectra of the samples were obtained by measuring their diffuse reflectance spectra in a Perkin Elmer UV/VIS 330 spectrophotometer and then converting to absorbance units by using the Kubelka-Munk theory. The average size and crystalline structure of the PbS nanoparticles were determined from transmission electron microscopy (TEM) measurements performed in a Jeol 2010 transmission electron microscope.
Results and Discussion
The XRD patterns of the three PbS-Na-X samples prepared at different temperatures are shown in Figure 1. In addition, at the bottom it is plotted the XRD pattern of the pure Na-X zeolite, which displays the diffraction lines of the faujasite zeolite (JCPDS # 12345). There are not big differences between the patterns of the pure and PbS loaded zeolites, indicating that the crystallinity of zeolite X is not affected by the synthesis process. The patterns of the PbS-Na-X samples do not display any PbS diffraction peak due to the small concentration of PbS in the zeolite matrix.
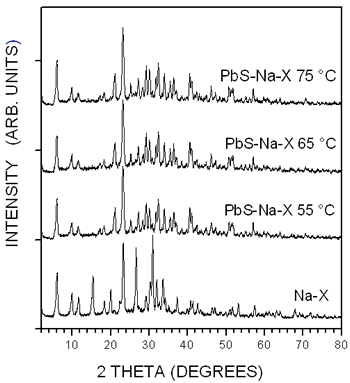
Figure 1. X-ray diffraction patterns of the unloaded Na-X zeolite and of the three PbS-Na-X samples.
In Figure 2 it is shown the TEM image of a zeolite crystallite of the sample prepared at 65ºC. In this image it is possible to observe particles smaller than 10 nm uniformly embedded in the crystallite and its edge with emerging nanoparticles. The nanoparticles have a very well-defined spherical shape.

Figure 2. TEM image of PbS-Na_X sample prepared at 65ºC.
The particle size distribution shown in the histogram of Figure 3 was obtained from this picture yielding an average size of 4.6 nm, much smaller than the exciton Bohr diameter in PbS.
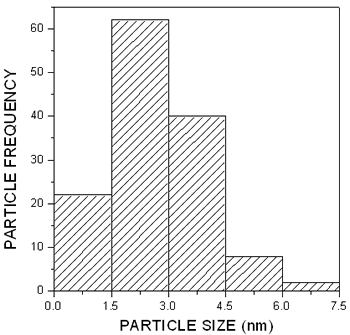
Figure 3. Particle size distribution obtained from the image in Figure 2.
The electron diffraction pattern, shown in Figure 4, was used for the determination of the crystalline structure of the nanoparticles and therefore for their identification.

Figure 4. Electron diffraction pattern of PbS nanoparticles in zeolite X.
The bright spots in this pattern define the interplanar distances of the nanoparticle crystalline lattice given in Table 1, where are also given the interplanar distances for the cubic PbS crystalline structure (Galena). The values of the interplanar distances of the nanoparticles crystalline lattice agree very well with those of the galena structure and therefore we can conclude that the nanoparticles embedded in the zeolite observed in the TEM image are PbS crystalline nanoparticles with cubic structure.
Table 1. Interplanar distances of PbS-Na-X sample, PbS bulk (Galena) and their corresponding crystalline lattice planes.
|
|
|
2.87
|
2.96
|
(200)
|
|
2.07
|
2.09
|
(220)
|
|
1.77
|
1.79
|
(311)
|
|
1.52
|
1.48
|
(440)
|
|
1.36
|
1.36
|
(331)
|
|
1.07
|
1.04
|
(440)
|
Another direct evidence of the formation of very small PbS particles in the zeolite matrix is given by the optical absorption spectra of the three PbS-Na-X samples, shown in Figure 5. The optical spectra of the samples prepared at 55 and 65ºC have an absorption onset at about 500 nm, meanwhile in the spectra of the sample prepared at 75ºC, the onset is observed at about 800 nm. The position of the absorption edge in the spectra of the three samples is strongly shifted to higher energies than the fundamental absorption edge of bulk PbS, indicating the great influence of quantum confinement as consequence of the small size of the PbS nanoparticles.
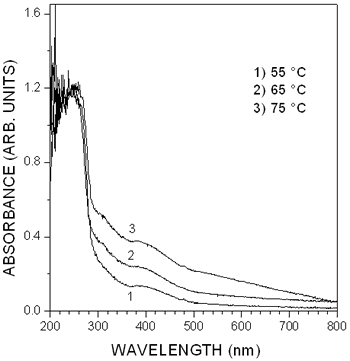
Figure 5. Absorption spectra of the PbS-Na-X samples measured at room temperature by diffuse reflectance spectroscopy.
In addition, the three optical spectra display two well-defined absorption bands with maximum at about 260 and 400 nm. Excitonic absorption bands at energy much higher than the energy band gap of bulk PbS have been observed in the absorption spectra of colloidal PbS particles with an average size of 4.3 nm stabilized with polyvinyl alcohol (PVA) [9]. The maximum of the absorption bands lie at about 600, 400 and 300 nm in the absorption spectra. Solving the Schroedinger equation with an infinite potential well and parabolic energy bands, it has been determined that the absorption bands were related to 1se-1sh, 1se-1ph and 1pe-1ph transitions, respectively. These exciton absorption bands have also been observed in the absorption spectra of PbS nanoparticles synthesized in polymers [12] and in Langmuir-Blodgett monolayers [13]. Thus, we can ascribe the origin of the absorption bands at 400 and 260 nm to 1se-1ph and 1pe-1ph transitions in PbS nanoparticles with average size of 4 nm, respectively. The shift in the absorption band at 260 nm in the spectra of the PbS-Na-X samples compared to 300 nm where it is observed the band due to the 1pe-1ph transition in PbS nanoparticles stabilized in PVA can be due to the influence of the zeolite matrix. In a recent paper we found that the 1se-1ph and 1pe-1ph transition appear at 280 and 390 nm in the optical spectra of PbS spherical nanoparticles in Zeolite A matrix, with an average size of 4.3 nm [6]. PbS clusters with an average size between 0.56 and 1.1 nm have also been synthesized in zeolite Y. The absorption spectra of this system display a single absorption peak at about 300 nm. The lack of the excitonic structure in the absorption spectra of PbS-Y system is explained as consequence of the weak exciton binding energy in narrow band gap semiconductors such as PbS.
The spherical shape of the PbS nanoparticles and its distribution in the Na-X zeolite matrix are characteristics very similar to the those of colloidal PbS nanoparticles stabilized in PVA reported in reference [9]. Since the average size of the nanoparticles, 4.6 nm, is larger than the size of the super cage in zeolite X, 1.3 nm, it can be concluded that the PbS nanoparticles are not encapsulated in the zeolite X cages but embedded uniformly in the zeolita matrix, as shown in the image of Figure 2. Even though the PbS nanoparticles are not located inside the regularly distribute zeolite cages, they exhibit interesting properties being their regular spherical shape and crystallinity the most significant. The crystalline quality of a semiconductor has been related to the appearance of excitonic absorption in its optical absorption [3]. Thus, the excitonic absorption observed in Figure 5 can be attributed to the good crystalline quality of the PbS nanoparticles, supported by the spot electron diffraction pattern shown in Figure 4.
Conclusions
In this work we have reported a simple chemical method based on ion exchange for the synthesis of PbS nanoparticles in zeolite Na-X. The PbS nanoparticles have spherical shape with an average size of about 4.0 nm. They have the crystalline structure of galena structure and are embedded uniformly in the zeolite matrix. The 1se-1ph and 1pe-1ph excitonic transitions observed in the absorption spectra of the PbS-Na-X system is explained in terms of the crystalline quality of the PbS nanoparticles.
Acknowledgements
We acknowledge Dr. R. Machorro from CCMC-UNAM for the diffuse reflectance measurements and the technical assistance of I. Gradilla-Martínez, F. Ruiz-Medina, M. A. Hernández-Landaverde, J. E. Urbina-Alvárez, R. Flores-Farias and P. Garcia. This work was financially supported by CONCyTEQ from Queretaro, México (Project number QRO-2003-C01-10419).
References
1. T. Trindade, P. O’Brien, and N. L. Pickett, “Nanocrystalline Semiconductors: Synthesis, Properties, and Perspectives”, Chem. Mater., 13 (2001) 3843-3858.
2. J. He, Y. Ba, C. I. Ratcliffe, J. A. Ripmeester, D. D. Klug, and J. S. Tsea, “The Nature of Encapsulated Silicon Nanoclusters in Zeolite Y”, Appl. Phys. Lett., 74 (1999) 830-832.
3. Wei Chen, Zhaojun Lin, Zhanguo Wang and Lanying Lin, “Some New Observation on the Formation and Optical Properties of CdS Clusters in Zeolite-Y”, Solid State Commun., 100 (1996) 101-104.
4. Wei Chen, Zhanguo Wang, Zhaojun Lin, Jiajun Qian, and Lanying Lin, “New Observation on the Formation of PbS Clusters in Zeolite-Y”, Phys. Lett., 68 (1996) 1990-1992.
5. R. Ochoa-Landín, M. Flores-Acosta, R. Ramírez-Bon, H. Arizpe-Chávez, M. Sotelo-Lerma and F. F. Castillón-Barraza, “Characterization of Cds Clusters in Zeolite-A Grown in Alkaline Solution” , J. Phys. Chem. Solids, 64 (2003) 2245-2251.
6. M. Flores-Acosta, M. Sotelo-Lerma, H. Arizpe-Chávez, F. F. Castillón-Barraza and R. Ramírez-Bon, “Excitonic Absorption of Spherical PbS Nanoparticles in Zeolite A”, Solid State Commun., 128 (2003) 407-411.
7. J. I. Pankove, “Optical Processes in Semiconductors”, (Dover Publications Inc., New York, 1971).
8. A.K. Dutta, T. Ho, L. Zhang and P. Stroeve, “Nucleation and Growth of Lead Sulfide Nano- and Microcrystallites in Supramolecular Polymer Assemblies”, Chem Mater., 12 (2000) 1042.
9. T.D. Krauss and F.W. Wise, “Raman-Scattering Study of Exciton-Phonon Coupling in PbS Nanocrystals”, Phys. Rev., B 55 (1997) 9860-9865.
10. T. Okuno, A. A. Lipovskii, T. Ogawa, I. Amagai and Y. Masumoto, “Strong Confinement of PbSe and PbS Quantum Dots”, J. Lumin., 89 (2000) 491-493.
11. R. S. Kane, R. E. Cohen, and R. Silbey, “Synthesis of PbS Nanoclusters within Block Copolymer Nanoreactors”, Chem. Mater., 8 (1996) 1919-1924.
12. Z., Suhua Wang, and S. Yang, “Synthesis and Characterization of PbS Nanocrystallites in Random Copolymer Ionomers”, Chem. Mater., 11 (1999) 3365-3369.
13. L. Song Li, L. Qu, L. Wang, R. Lu, X. Peng, Y. Zhao and T. Jin Li, “Preparation and Characterization of Quantum-Sized PbS Grown in Amphiphilic Oligomer Langmuir-Blodgett Monolayers”, Langmuir, 13 (1997) 6183-6187.
14. L. Guo, K. Ibrahim, F.Q. Liu, X.C. Ai, Q.S. Li, H.S. Zhu and Y.H. Zou, “Transient Optical Properties of Novel PbS Nanoparticles Coated with 2,6-O-diallyl-b-CD”, J. Lumin., 82 (1999) 111-114.
15. A. Martucci, P. Innocenzi, J. Fick and J.D. Mackenzie, “Zirconia-Ormosil® Films Doped with PbS Quantum Dots”, J. Non-Cryst. Solids, 244 (1999) 55-62.
Contact Details
|