| The environmentally friendly nature of supercritical fluids (SCFs) such as supercritical carbon dioxide (scCO2) has led to the exploration of their use in a range of materials applications. Recently, a number of research groups, including one at Liverpool University, have used the unique physical characteristics of scCO2 (and other fluids) in order to produce well-defined nanostructures for potential applications in areas such as inorganic catalyst supports, biomaterials and metal foams. High Internal Phase Emulsions (HIPEs) Using emulsion templating, a versatile method for producing porous materials, Cameron and colleagues at the University of Durham, UK, have developed improved techniques for producing cross-linked polystyrene materials by polymerising water-in-oil (W/O) high internal phase emulsions (HIPEs). The resulting materials or ‘polyHIPEs’ have well-defined porous structures that are skeletal replicas of the original emulsions. The reverse approach is also possible. The polymerisation of oil-in-water (O/W) HIPEs provides a direct route to a range of porous hydrophilic materials, including biocompatible hydrogels. However, one drawback is that O/W HIPE techniques are very solvent intensive because the oil phase volume fraction is typically greater than 75%. Also, it may be difficult to remove the organic template phase from the pores after polymerisation. Use of Supercritical Fluids to Produce HIPEs At Liverpool University, we have overcome these problems using supercritical fluids. We have developed a method for producing hydrophilic polyHIPEs in the absence of any organic solvents by directly polymerising high internal phase CO2-in-water emulsions (C/W HIPEs), figure 1. Using this new process, removal of the template phase is simple because the CO2 reverts to the gaseous state upon depressurisation. This method has been used for the preparation of cross-linked polyacrylamide and poly(hydroxyethyl acrylate) materials with pore sizes of a few micrometres or so. One of the university’s future goals is to extend this method to the preparation of materials with structural control on the nanometre scale. The university is also investigating techniques for using variations in CO2 density to ‘fine tune’ structure. The complete absence of any organic solvents, during both production and purification, may make this method attractive for producing biocomposite materials. | 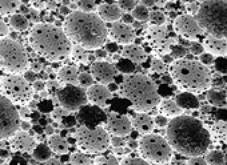
| | Figure 1. Templated C/W HIPE synthesised from acrylamide and N,N-methylene bisacrylamide. Approximate image size 220x220µm, average pore diameter is 3.9µm, total pore volume is 3.9cm3/g. No organic solvents were used in the preparation, only water and CO2. | Nanoscale Casting Using Super Critical Fluids Wakayama and colleagues at the Toyota Central R&D Laboratories, Japan, have developed another approach to the preparation of porous materials. Nanoscale casting using supercritical fluids (NC-SCF) takes advantage of several of the important physical properties associated with SCFs. For example, nanoporous silica can be prepared by dissolving silica precursors (e.g. TEOS) in scCO2 before attaching the precursors to activated carbon moulds. The activated carbon is then removed and the samples coated with platinum or other materials. The Japanese group found that they could replicate not only macroscopic shapes such as fibres, but also porous structures on the nanometre scale. Perhaps the key advantage of using a supercritical fluid in this process is the unusually low viscosity and absence of surface tension. This means that even complex nanostructures can be replicated and preserved after solvent removal in a way that might be difficult to achieve with conventional liquid solvents. Lithography Another process that can benefit from low solvent viscosity is lithography. Supercritical fluids tend to have much lower viscosities than liquid solvents. At Cornell University USA, Ober has developed diblock co-polymer resists for 193 nm wavelength lithography using scCO2 as the developing solvent. Using this method, lithographic resolutions as low as 200 nm can be achieved, figure 2, as a result, in part, of the interfacial segregation behaviour exhibited by the block copolymers resists. The Cornell team believes that scCO2 development could play a key role in the fabrication of high aspect ratio features because of the absence of surface tension forces. | 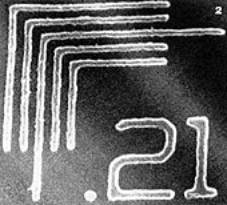
| | Figure 2. Electron image of a 193nm wavelength photolithographic image developed using scCO2. | Ober's research focuses on image development using supercritical CO2. In fact, the viscosity of CO2 is also low in the liquid state. It has extremely low surface tension and surface energy, lower than even many fluorocarbons, which gives the solvent remarkable wetting properties. Using this fact, Carbonell and DeSimone at the NSF Centre for Environmentally Responsive Solvents and Processes, US, have developed techniques for spin coating polymer resists onto silicon wafers directly from liquid CO2 A combination of these two technologies could result in a completely new photolithographic process that uses no organic or aqueous solvents whatsoever, either in the coating or developing steps. Metal Nanoparticles Given the enormous level of interest in the field of metal nanoparticles, it is perhaps not surprising that supercritical fluid solvents have been explored in this context. The production and processing of nanoparticles is an area in which fine control over solvent properties may offer distinct advantages. For example, Schiffrin and co-workers at Liverpool University have shown that the variable density associated with SCFs may be exploited in the size fractionation of functionalised metal nanoparticles. The actual production of nanoparticles in SCF solvents offers advantages such as rapid solvent separation, accelerated reaction rates (owing to high diffusivities) and the possibility of depositing particles in situ in porous materials, thereby taking advantage of the unique properties of the SCF phase. However, one problem with conventional approaches to nanoparticle production and processing is that they are not always directly transferable. For example, alkanethiol-capped nanoparticles were found not to disperse readily in scCO2 owing to the very low van der Waals forces and polarisability exhibited by this solvent. This problem has been addressed by Johnston and Korgel at the University of Texas, USA, by producing fluorocarbon-capped metal nanoclusters from soluble organometallic precursors by arrested precipitation in scCO2, figures 3a and 3b. The production and processing of metal nanoparticles capped with fluorinated ligands (e.g. 1H,1H,2H,2H-perfluorodecanethiol) could prove important in applications such as memory storage in which a low dielectric constant coating material may help to insulate the active charge storing devices. | 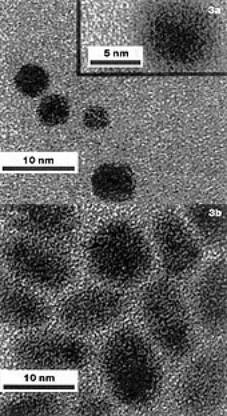
| | Figure 3. TEM images of materials prepared by thereduction of soluble organometallic precursors in scCO2. (a) Iridium nanocrystals coated with C6F13C2H4SH and (b) platinum nanocrystals coated with C6F13C2H4SH. | The Future for Supercritical Fluids SCF solvents show great potential for the creation of nanostructures. As recognised for many years in the field of aerogels, surface tension and capillary forces can be highly destructive at the nanoscale. Also, complex supramolecular structures are typically formed in solution, and the self-assembly process is invariably influenced by solvent variables such as density, dielectric constant and polarisability. All of these variables can be fine-tuned in compressible SCF solvents, and the control of molecular self-organisation is likely to be one of the new frontiers for this technology. |