Introduction The nickel oxide catalyst has been widely used in petrochemical industry, for example the synthesis of olefin gas, reforming reaction of methane and so on. Technique used to synthesize spherical oxide particles is important in designing new ceramic and catalytic materials. Among the numerous methods suggested for synthesizing spherical particles, the sol-gel method is currently the most promising. For preparation of NiO and other metal oxides, Korošec et al. [1] prepared nanosize NiOx films via the sol-gel method through alkaline hydrolysis of nickel hydroxide. The electro chromic response and electrochemical stability of Ni-films synthesized by sol-gel route largely depended on the preparation conditions and the precursor used. Ogihara et al. [2] synthesized a monodisperse ZrO2 powder through alkaline hydrolysis of zirconium butoxide. The morphology of the resulting particles and their size distribution strongly depend on the concentration of the initial alkoxide and water/alkoxide ratio. Li and Messing [3] obtained spherical ZrO2 particles by adding excess isopropyl alcohol to a partially hydrolyzed concentrated solution of zirconyl salts; however, the resulting oxide powder had a broad size distribution. Sanz et al. [4] selected sol-gel route to produce glasses for optical applications because its low temperature processing is appropriate for the thermal stability of organic phases. Colomer and Anderson [5] designed nano-, micro- and meso-porous SiO2 xerogels of high porosity and small pore size using sol-gel route with a water:TEOS molar ratio of 83, aiming to obtain proton exchange electrolytes for proton exchange membrane fuel cells (PEMFCs). Sol-gel process offers a narrow pore-size distribution of the final product. In this process, the pH of the colloidal silica not only influences hydrolysis and condensation rates but also influences the packing properties of the particles obtained. Iler [6] showed that when the sol was acidified and dried, the gel had higher density than that of which the sol was not acidified. Since porosity is directly related to packing density, if one can control the packing process, membranes having various porosities and average pore sizes can be achieved. Gao et al. [7] prepared an ultra fine nickel powder and studied its catalytic dehydrogenation activity. They found that at pH 9-10 the ultra fine nickel powder having high purity was obtained. From the above mentioned literature, it can be concluded that using sol-gel method to produce catalyst helps to save energy and provides high purity product with uniform size distribution. In this work, the effect of different bases used for preparing NiO catalyst via sol-gel route is presented. The special attention was focused on studying the properties of catalyst obtained from different bases. Experimental NiO was prepared by sol-gel method under basic catalyzed system. The following analytical grade reagents were used: Ni(NO3)2·6H2O, NiSO4·6H2O, NiCl2·6H2O, NaOH and KOH. Nickel salts were dissolved in deionized water to form solutions with varied concentrations of 0.3, 0.5 and 0.7 M. Then 2 M of NaOH or KOH, was added drop-wise into the solutions and stirred continuously at room temperature until pH approached 9. Subsequently, green precipitate was filtered out and rinsed with deionized water until pH was about 7. After dried at 80°C for overnight, nickel hydroxide gel was formed following these reaction steps: Ni(NO3)2 + 2NaOH → Ni(OH)2 + 2NaNO3 (1) Ni(NO3)2 + 2KOH → Ni(OH)2 + 2KNO3 (2) NiSO4 + 2NaOH → Ni(OH)2 + Na2SO4 (3) NiSO4 + 2KOH → Ni(OH)2 + K2SO4 (4) NiCl2 + 2NaOH → Ni(OH)2 + 2NaCl (5) NiCl2 + 2KOH → Ni(OH)2 + 2KCl (6) Received Ni(OH)2 was converted to NiO by calcining at varied temperatures of 350, 550, and 750°C for 1 h. Characterization The products obtained were characterized by a Powder X-ray diffraction (XRD: Phillips, Analytical X-ray B.V. PW 1830/40), Scanning Electron Microscopy (SEM: JEOL, JSM 5410), and BET Surface Area Analyzer (Quantachrome Autosorb I) using BET-multipoint method. Results and Discussion The products obtained from synthesis by using NaOH and KOH were grey-black powders. The XRD patterns of NiO powders obtained from 0.7 M of Ni(NO3)2 and NaOH calcined at different temperature are shown in Figure 1(a). When the product was calcined at 350, 550 and 750°C, the crystal phase of NiO was observed from the three main peaks at 2θ about 38, 43 and 62.5 degrees. From the height of main peaks it is clearly seen that the product calcined at 750°C had the largest crystal size compared to the others because at higher temperature the crystal has more energy for the growing stage. When NiCl2 and NiSO4 were used instead of Ni(NO3)2, the XRD patterns of NiO powders calcined at 750°C are shown in Figure1(b) compared with that of Ni(NO3)2. It can be concluded that the received NiO from Ni(NO3)2 had the largest crystal size followed by those of NiCl2 and NiSO4, respectively. This is also confirmed by the SEM pictures shown in Figure 2. 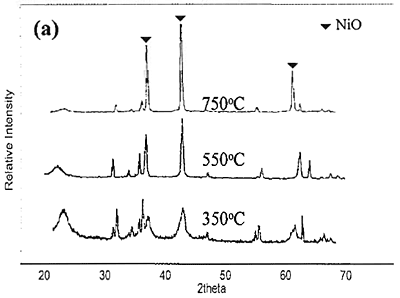
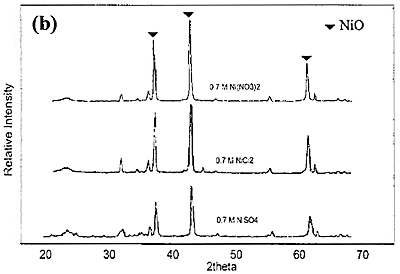
Figure 1. XRD patterns of NiO particles obtained from 0.7 M of different nickel salts and NaOH. (a) using Ni(NO3)2 and calcined at 350, 550 and 750°C. (b) using NiCl2 and NiSO4 and calcined at 750°C 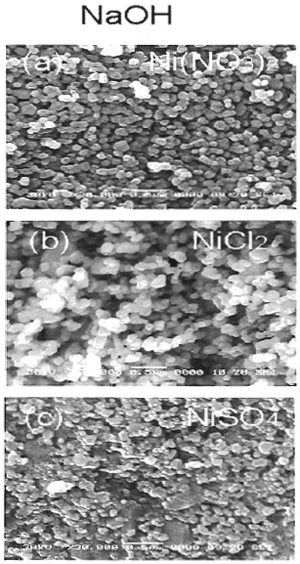
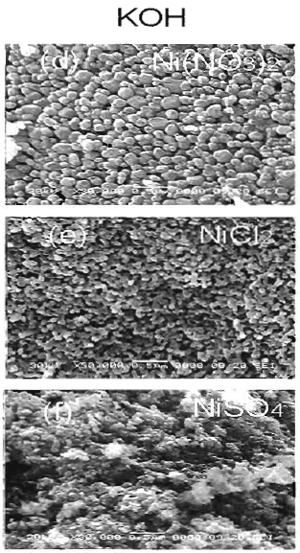
Figure 2. SEM micrographs of NiO powder obtained by hydrolysis of 0.7 M of Ni compounds and calcined at 750°C for 1 h (a), (b) and (c) using NaOH while (d), (e) and (f) using KOH. The effect of base type (NaOH and KOH) on the size of NiO particle was shown in Figures 3 and 4. A slightly bigger NiO particles (0.12 μm) were obtained when NaOH was used, compared to that of KOH (0.08 μm). Sodium and potassium are in the same group of IA in the periodic table, but sodium molecule is smaller than potassium molecule. Therefore, potassium has higher reactivity to form ion and reacts with nickel precursors to form Ni(OH)2. 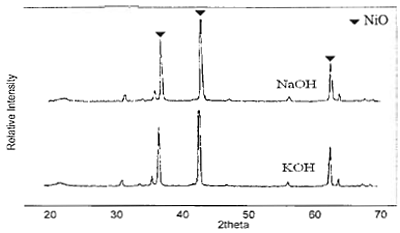
Figure 3. XRD patterns of NiO particles obtained from 0.7 M of Ni(NO3)2 and NaOH and KOH calcined at 750°C for 1 h. 
Figure 4. SEM micrographs of NiO obtained from 0.5 M of Ni(NO3)2 and calcined at 750°C for 1 h (a) using NaOH (b) using KOH. From SEM micrographs, it was found that synthesized NiO particles were uniformly distributed and had spherical shape as shown in Figure 4. The BET surface areas of products are summarized in Table 1, which shows that NiO particles prepared from Ni(NO3)2 and NiCl2 had specific surface areas in the same range of 5.2 – 5.5 m2/g that was about 2 times higher than that from NiSO4. Moreover, use of NaOH yielded slightly larger surface area than that of KOH. Table 1. BET surface area of products calcined at 750°C | | | 0.7 M of Ni(NO3)2 | NaOH | 5.5 | | KOH | 5.4 | | 0.7 M of NiCl2 | NaOH | 5.4 | | KOH | 5.2 | | 0.7 M of NiSO4 | NaOH | 3.1 | | KOH | 2.9 | Conclusion Use of different nickel precursors and bases yielded the NiO particles with different sizes. NiO particle from Ni(NO3)2 was the biggest size followed by NiO from NiCl2 and NiSO4 respectively. In addition, use of NaOH yielded slightly bigger NiO particle than that of KOH. The NiO crystal size increased with increase of calcined temperature and concentration of nickel salts. In this work, using of Ni(NO3)2 and NaOH was found to be the appropriate reactants for synthesis of nickel oxide because of received product having uniform size distribution compared with the others. References 1. R.C. Korošec and P. Bukovec, “The role of thermal analysis in optimization of the electrochromic effect of nickel oxide thin films, prepared by sol-gel method: part II”, Thermochemica, 410 (2004) 65-71. 2. T. Ogihara, N. Mazutani and M. Kato, “Processing of monodispersed ZrO2 powders”, Ceram. Int., 13 (1987) 35-40. 3. M. Li and G.L. Messing, Ceramic Powder Science III, Westerville (Ohio): Am. Ceram. Soc., 129 (1990). 4. N. Sanz, P.L. Baldeck and A. Ibanez, “Organic nanocrystals embedded in sol-gel glasses for optical applications”, Synthetic Metals, 115 (2000) 229-234. 5. M.T. Colomer and M.A. Anderson, “High porosity silica xerogels prepared by a particulate sol-gel route: pore structure and proton conductivity”, Journal of Non-Crystalline Solids, 290 (2000) 93-104. 6. P.K. Iler, The Chemistry of Silica, Wiley Interscience, New York, 1979. 7. J. Gao, F. Guan, Y. Zhao, W. Yang, Y. Ma, X. Lu, J. Hou and J. Kang, “Preparation of ultrafine nickel powder and its catalytic dehydrogenation activity”, Materials Chemistry and Physics, 71 (2001) 215-219. Contact Details | Chatchawan Sookman
Department of Chemical Engineering
Kasetsart University
50 Paholyothin, Jatujak
Bangkok
Thailand, 10900 E-mai: [email protected] | Paisan Kongkachuiychay
Department of Chemical Engineering
Kasetsart University
50 Paholyothin, Jatujak
Bangkok
Thailand, 10900 | |