May 2 2017
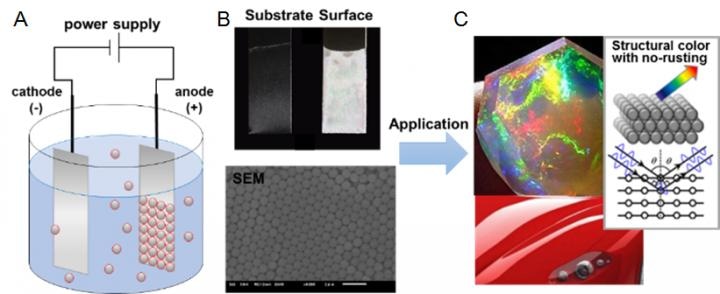 A) Illustration of the electrophoretic deposition (EPD) of sulfone-containing nano-latex shows negatively charged nano-particles in suspension migrating towards the positive electrode. B) The anode coated with aligned nano-particles (top: electrode, bottom: SEM image. Scale bar, 2000 nm). C) Images of application example and the light reflection by the colorless nano-particles. (Credit: NITECH)
A) Illustration of the electrophoretic deposition (EPD) of sulfone-containing nano-latex shows negatively charged nano-particles in suspension migrating towards the positive electrode. B) The anode coated with aligned nano-particles (top: electrode, bottom: SEM image. Scale bar, 2000 nm). C) Images of application example and the light reflection by the colorless nano-particles. (Credit: NITECH)
A novel and simple coating process to color metals has been invented by polymer chemists at Nagoya Institute of Technology in Japan. This invention helps saving the energy and leads to higher performance.
Electrophoretic deposition refers to a standard industrial method employed for coating material and is particularly used for rust prevention. However, the existing methods need an expensive and complex process requiring three coating steps, adding time and cost. Professor Akinori Takasu and his team report novel non-ionic polymers capable of being used with electrophoretic deposition, thus simplifying the coating to only a single step and bringing about a major reduction in energy demands.
The inclusion of a specific chemical group to the non-ionic polymer molecule is considered to be the key to the discovery.
It was accidentally found in a project designing a new material for dental implant. When a non-ionic polymer had a sulfonyl group, it moved towards the anode in electrophoresis.
Professor Akinori Takasu, Nagoya Institute of Technology
The research team earlier demonstrated that the resulting coating becomes extremely thick when electrophoretic disposition is applied at low voltages. Incorporating a set of findings brought about the possibility to skip multiple coating processes on a metal for rust resistance. However, it is essential that the coat come in any desired color for commercial purposes. Takasu and his team studied the behavior of the color properties of non-ionic polymers in water after being applied as coating.
Our breakthrough was to include this non-ionic polymer into nano-particles. The new particles show structural color like opal stones, a.k.a. colorless color. The wavenumber of the particle should be controllable by changing the size of the particles used to coat the surface which determines the color emitted.
Professor Akinori Takasu, Nagoya Institute of Technology
Takasu studied that it is difficult to control the size of the particles even though he could effortlessly react the non-ionic polymers with the sulfonyl group. Takasu and his team, as part of this study, produced the size control technology and prepared the particles by soap-free emulsion copolymerization, which constantly gave nanoparticles 300 nm in size as an example. This was followed by oxidizing the particles in water in order to produce the sulfonyl group. Electrophoretic deposition was finally applied to coat steel. Electron microscopic images established the fact that the particles covered the steel uniformly in a honeycomb pattern.
I expect our study will lead to a new type of electrophoretic painting that can be applied to any coating technologies like cars and fibers.
Professor Akinori Takasu, Nagoya Institute of Technology
This technique helps overcome issues such as color fading and damage from UV radiation due to structural coloring, and hence it will further be employed for a wider application of electrophoretic dispersion.
The article "Electrophoretic non-ionic nano-spheres (latexes) for structural coloring" was featured in Polymer at DOI: 10.1016/j.polymer.2017.04.019