.jpg) By Susha Cheriyedath, M.Sc.Jan 10 2022Reviewed by Skyla Baily
By Susha Cheriyedath, M.Sc.Jan 10 2022Reviewed by Skyla BailyA group of researchers recently published a paper in Materials that demonstrated the feasibility of using pH-stable luminescent and reusable sensors for detecting phosphate in a highly selective manner.
Study: Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Image Credit: StudioMolekuul/Shutterstock.com
Phosphate sensors are keenly studied for their role in monitoring the aquatic environment, specifically phosphate. The presence of phosphate can lead to algal blooms, which makes the monitoring of phosphate essential for evaluating and understanding eutrophication.
However, the detection of phosphate is currently difficult owing to the chemical characteristics of the substance. Thus, researchers are focusing on luminescent coordination polymer particles (CPP) that can sensitively and selectively respond against the phosphate ions in the aquatic environment.
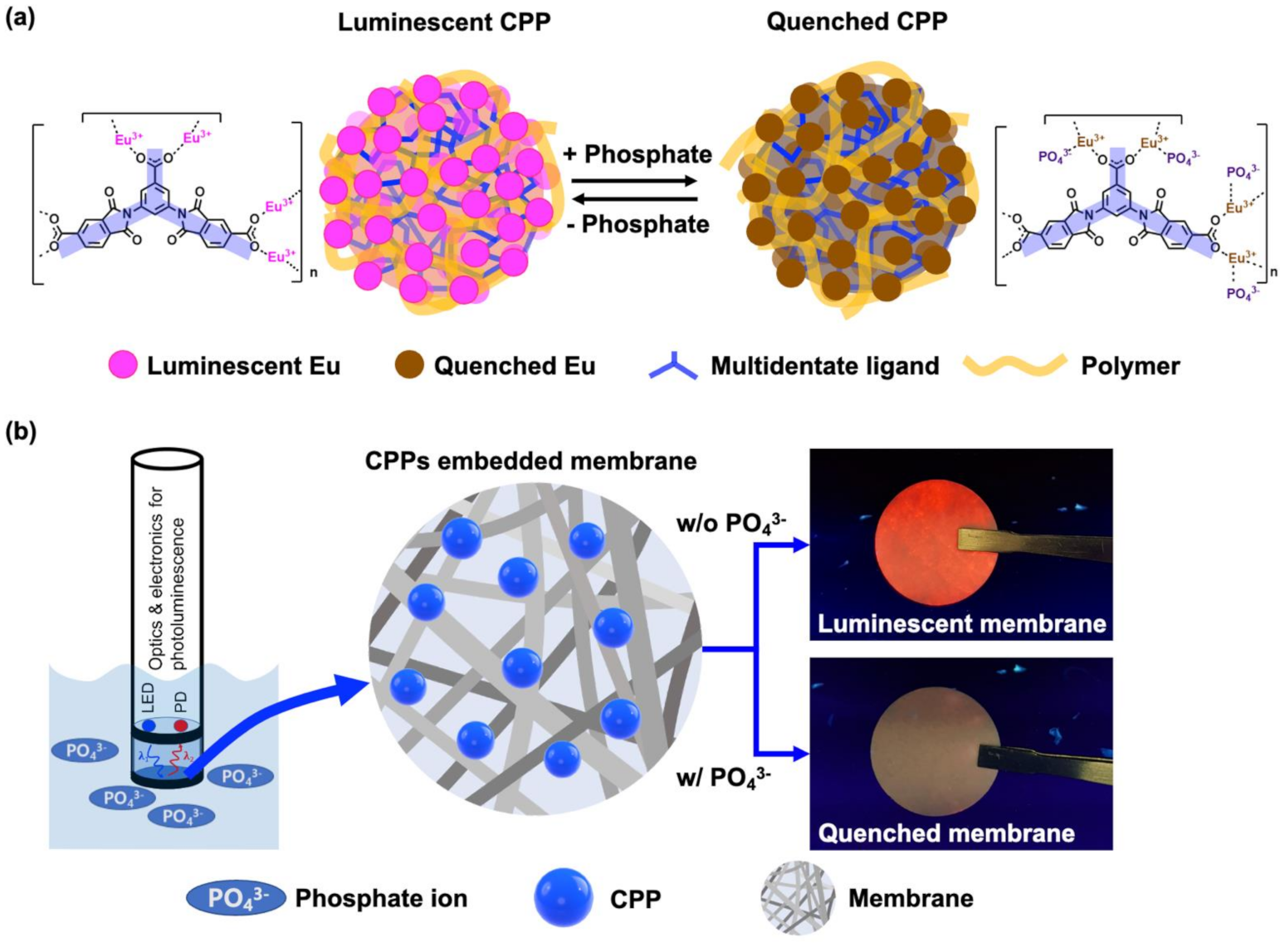
(a) Schematic illustration of the luminescent “turn-off” sensing based on CPP for detection of phosphate. (b) Conceptual configuration of phosphate sensor composed of light source, detector, and membrane containing CPPs as sensing materials. Image Credit: Kim, D.Y et al., Polymers
Significance of Phosphate Detectors
Algal blooms commonly found in different aquatic environments often show a complex behavior owing to different factors such as nutrients (phosphate and ammonia). However, harmful algal blooms (HAB) such as Oscillatoria and Microcystis produce toxins that can adversely impact both humans and aquatic life.
Thus, seamless monitoring of HABs and applying proper algae removal treatments are necessary for conserving the aquatic environment. Although the other nutrients responsible for the growth of HAB can be monitored by using different field-deployable sensors, seamless detection and monitoring of phosphate remain a challenge.
Phosphate detection is a complicated process that can only be performed in high temperature and pressure conditions. The factor limits the application of field-deployable phosphate sensors without using chemical reagents. Different forms and large sizes of phosphate ions, and high solvation energy of phosphates are the other factors that make phosphate detection challenging.
These factors have increased the focus towards CPPs due to their tunability and substantially large surface area. Thus, researchers in the present study intended to demonstrate that a CPP-based photoluminescence phosphate sensor can offer a high selectivity, sensitivity, and cover a large range of pH required for phosphate detection.
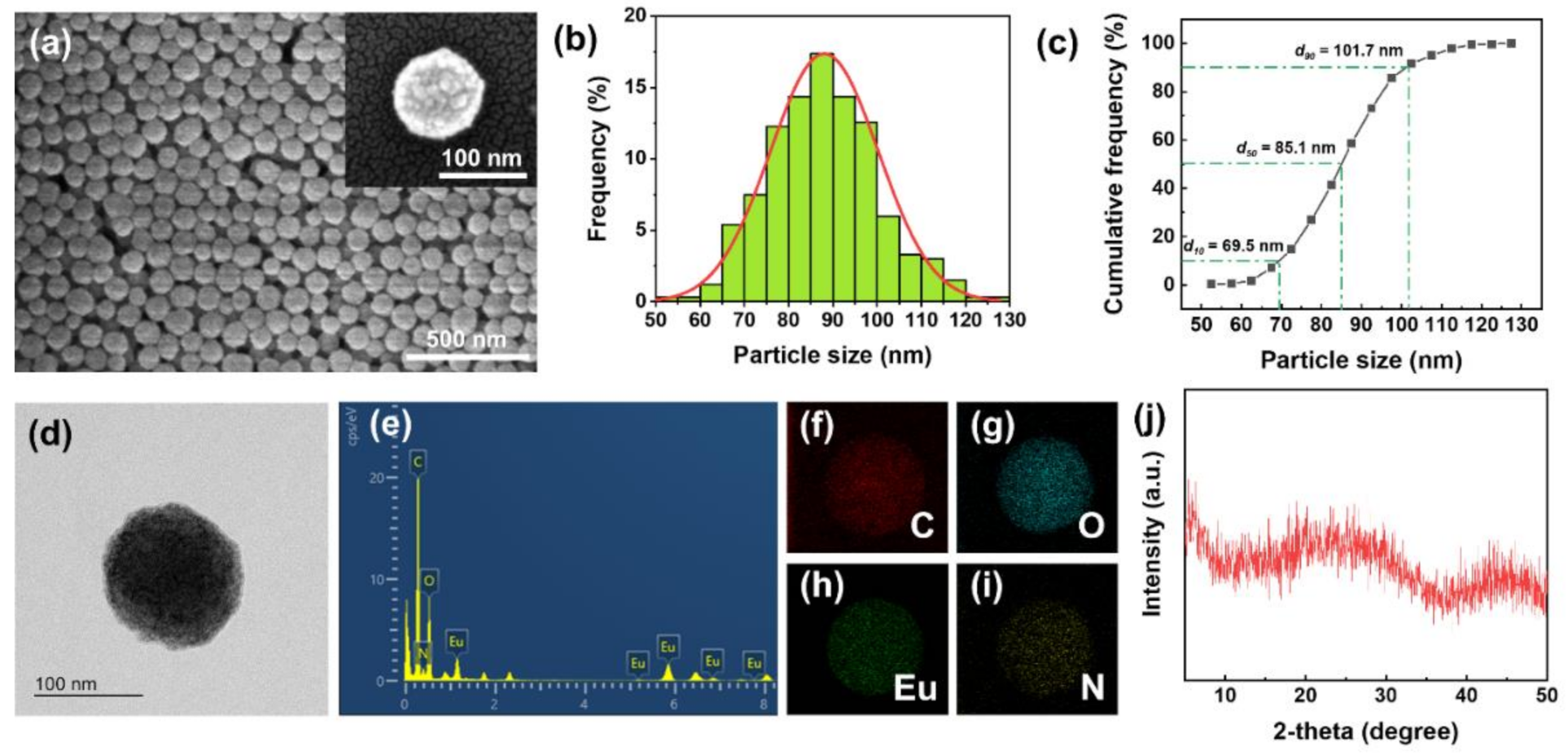
(a) SEM images, (b) size histogram, (c) cumulative frequency distribution, (d) TEM image, (e) EDX spectrum, (f–i) EDX elemental mapping, and (j) XRD spectrum of Eu-TCA. Image Credit: Kim, D.Y et al., Polymers
The Study
The CPP used in this study is more durable in the aquatic environment than the other luminescent frameworks. Additionally, this CPP can be produced in the form of nanoparticles for embedding them in a porous membrane. Luminescent CPP for this study has been mainly prepared using polyvinylpyrrolidone (PVP) and EuCI3.6H2O.
Other reagents used in the preparation include H2SO4, potassium phosphate, and ethanol, dimethylformamide (DMF), and 2,20-(5-Carboxy-1,3-phenylene) bis (1,3-dioxoisoindoline-5-carboxylic acid) (TCA). The Eu-TCA was obtained successfully by regulating the synthetic parameters such as pH and reaction temperature. pH control and the addition of PVP impacted the crystallinity and shape of CPPs. The Eu-TCA was embedded in the glass microfiber filters (GMF) for phosphate detection.
Transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDX) and field emission scanning electron microscopy (SEM) were used for analyzing the surface microstructure and morphology of the Eu-TCA.
Rigaku diffractometer was used to obtain the powder X-ray diffraction (PXRD) data. Nicolet iS10 Fourier transform infrared (FTIR) spectrometer and K-Alpha+ spectrometer equipped with a micro-focused monochromatic Al Kα X-ray source were used for obtaining the FTIR spectra and X-ray photoelectron spectroscopy (XPS) measurements, respectively.
A spectrophotometer was used for studying the photoluminescence of the Eu-TCA dispersion, while a microplate reader was used for investigating the phosphate sensing performance of the GMF/Eu-TCA by measuring the luminescence intensity of the microplate containing the GMF/Eu-TCA. The emission and excitation wavelengths were selected as 615 nm and 260 nm, respectively.
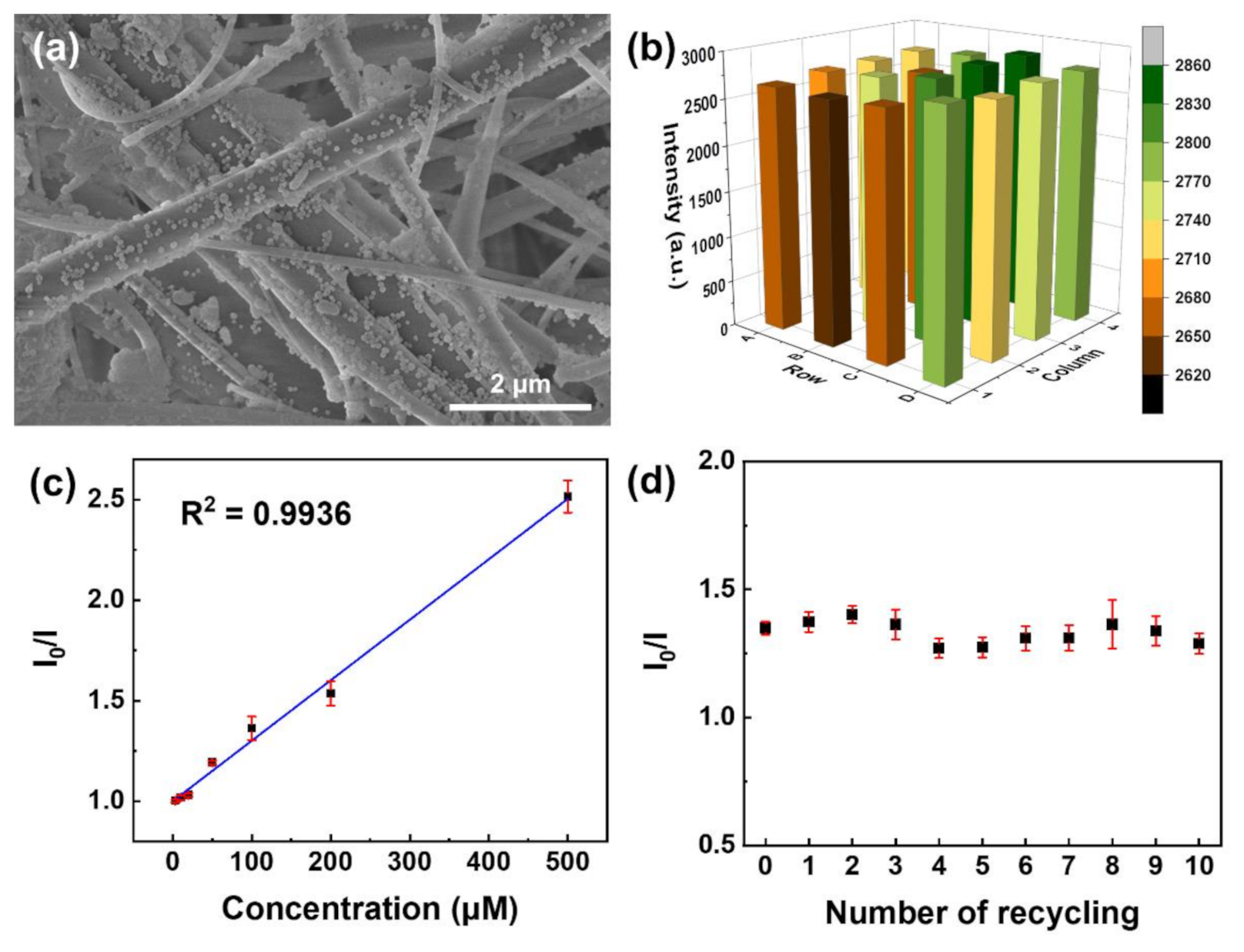
(a) SEM image of Eu-TCA/GMF. (b) Luminescence intensity of 4 × 4 wells of microplate containing the independently fabricated Eu-TCA/GMFs. (c) Stern–Volmer plot of the luminescence quenching of Eu-TCA/GMF (n = 7) as a function of phosphate concentration. (d) The luminescence intensity of Eu-TCA/GMF (n = 7) after ten runs of recycling. The concentration of phosphate is 100 μM. Image Credit: Kim, D.Y et al., Polymers
Observations
Observations from the TEM and SEM analysis indicated the formation of amorphous coordination polymers owing to the early short-range regularity. The simultaneous existence of dynamic and static luminescence quenching phenomena was observed while investigating the phosphate detection performance of the Eu-TCA. The FTIR data strongly supported the reorganization of coordination bonds and phosphate binding around Eu3+ metal centers in the Eu-TCAs.
Observations from XPS analysis were similar to that of the FTIR analysis, based on which the researchers concluded that GMF/Eu-TCA could be used as a reusable phosphate sensor. Relative standard deviation observed in the luminescence intensity indicated the reproducibility of batch-to-batch fabrication of GMF/Eu-TCA.
The sensing ability of the GMF/Eu-TCA was better or comparable with the other phosphate detection methods based on the limit of detection (LOD). Moreover, observations also indicated that GMF/Eu-TCA is suitable for detecting phosphate in a large pH range. Furthermore, the proposed phosphate detection platform was found feasible for use in real aquatic environments.
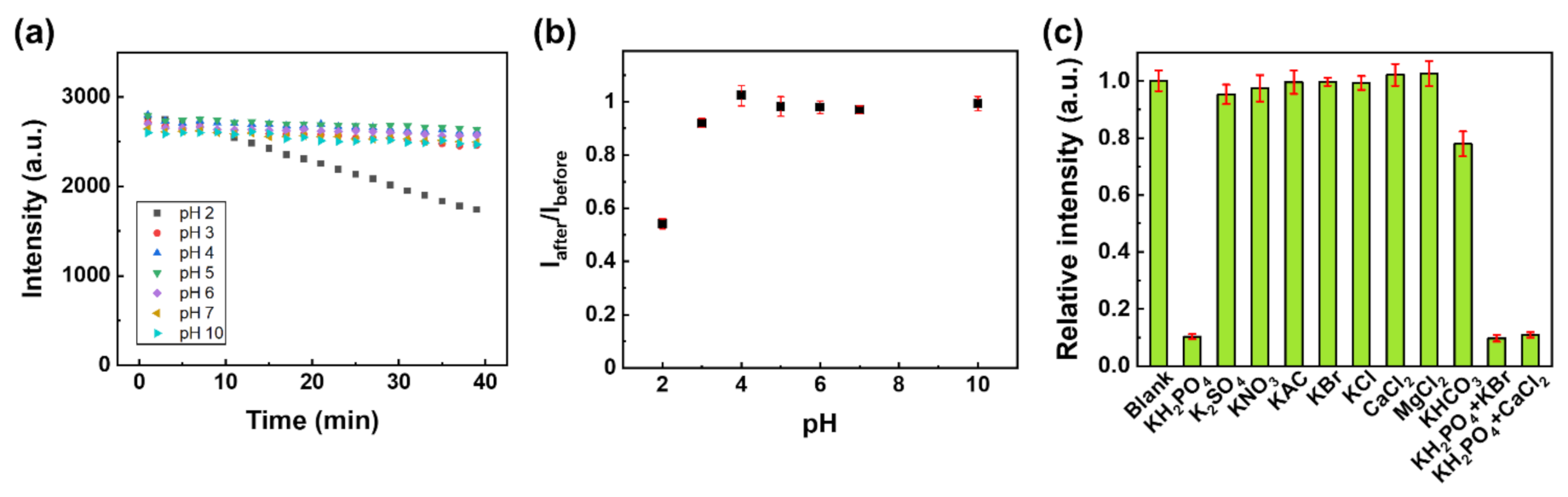
(a) The luminescence intensity of Eu-TCA/GMF as a function of time under various pH conditions. (b) Iafter/Ibefore of Eu-TCA/GMF (n = 6) as a function of pH, where Ibefore is the luminescence intensity before exposure to the pH solution and Iafter is the luminescence intensity after exposure to the pH solution. (c) The luminescence intensity of Eu-TCA/GMF (n = 6) immersed in phosphate and other various ionic solutions (1 mM). Image Credit: Kim, D.Y et al., Polymers
Future of CPP-Based Phosphate Detectors
The study demonstrated that luminescent nanoparticles such as Eu-TCAs can be synthesized as CPPs for detecting phosphates through precise coordination interactions. The phosphate detection sensor can promote chemical stability under different pH conditions. Hence, a membrane consisting of luminescent nanoparticles can be equipped in a probe-type photoluminescent sensor and function without any chemical activation process or redox chemicals.
Source
Kim, D.Y., Kim, D.G., Jeong, B. et al. Reusable and pH-Stable Luminescent Sensors for Highly Selective Detection of Phosphate. Polymers 2022, 14, 190. https://www.mdpi.com/2073-4360/14/1/190
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.