Tokyo Tech scientists showcased that donor doping within a mother material containing disordered intrinsic oxygen vacancies, as opposed to the commonly employed acceptor doping in vacancy-free materials, significantly amplifies the conductivity and durability of perovskite-type proton conductors within the temperature range of 250–400 ℃.
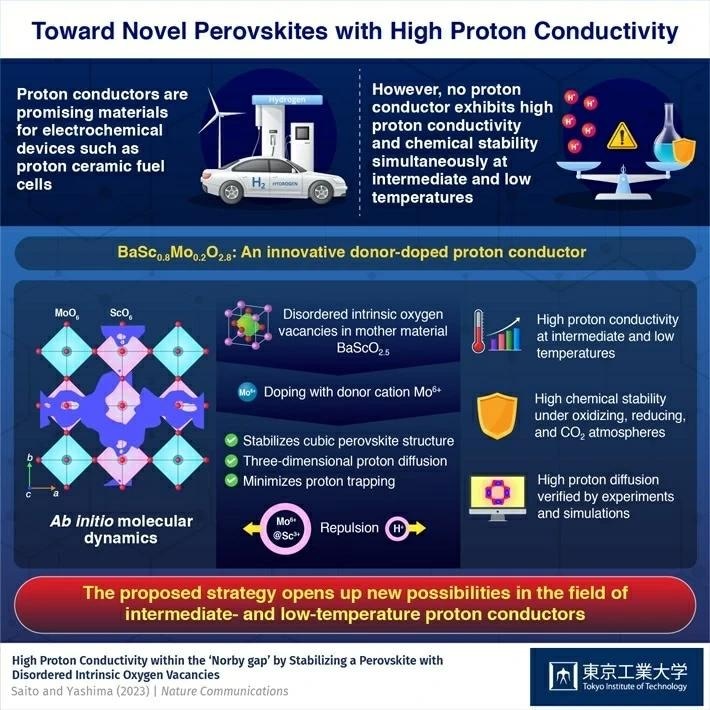
Image Credit: Tokyo Institute of Technology
For instance, conductivity reached 10 mS/cm at 320 ℃. This pioneering method charts a fresh pathway for designing proton conductors suitable for fuel cells and electrolysis cells.
Numerous nations worldwide are advocating for the advancement of sustainable energy technologies, among which protonic ceramic (or proton-conducting) fuel/electrolysis cells (PCFCs/PCECs) stand out prominently. These devices possess the capability to convert chemical energy into electricity and vice versa directly, emitting zero emissions, especially at low or intermediate temperatures.
This quality renders them highly appealing for diverse emerging applications, notably as next-generation distributed power sources. Additionally, unlike other fuel cells and electrolyzers, PCFCs/PCECs operate without the need for precious metal catalysts or costly heat-resistant alloys.
To date, no reports have surfaced regarding proton conductors that exhibit both high conductivity and exceptional stability within the temperature range of 250–400 ℃. Termed the "Norby gap," this challenge has persisted, prompting scientists to embark on a prolonged quest for materials capable of surpassing this limitation.
Given this context, Professor Masatomo Yashima and Mr. Kei Saito from the Tokyo Institute of Technology (Tokyo Tech), Japan, have introduced a groundbreaking strategy that holds the potential to transform the landscape of proton conductor design and development. Their research outcomes have been published in the esteemed multidisciplinary journal Nature Communications.
The scientists addressed a primary limitation of contemporary perovskite-type proton conductors. These materials adhere to the formula A2+B4+O3, featuring larger cations represented by A and smaller ones by B. Typically, a prevalent approach to boost proton conductivity in these perovskites involves introducing an acceptor dopant—a cation denoted as M3+ possessing a valence lower than that of B4+.
These "impurities" generate oxygen vacancies within the resulting crystalline lattice, consequently augmenting proton conductivity. Yet, this method introduces a challenge referred to as "proton trapping." This phenomenon occurs when protons become ensnared by the acceptor dopant M3+, which possesses an effective negative charge compared to the host cation B4+, leading to electrostatic attraction.
To circumvent this challenge, the researchers shifted their focus to BaScO2.5. This particular perovskite possesses inherent oxygen vacancies within its crystal structure, facilitating donor doping. Consequently, the team introduced the donor dopant Mo6+ into BaScO2.5, resulting in the creation of BaSc0.8Mo0.2O2.8, commonly denoted as "BSM20."
Contrary to the conventional acceptor doping approach, donor doping can reduce proton trapping effect through the electrostatic repulsion between protons and the donor Mo6+ cations, which have a higher valence than the host cation Sc3+. This, in turn, leads to high proton conduction.
Masatomo Yashima, Professor, Tokyo Institute of Technology
After conducting a sequence of experiments and employing sophisticated simulation methods, the researchers substantiated that BSM20 indeed showcased remarkably high proton conductivity within the Norby gap at intermediate and low temperatures.
Furthermore, donor doping contributed to stabilizing a cubic perovskite-type structure, facilitating efficient three-dimensional proton conduction across the material. Significantly, BSM20 displayed exceptional stability under various atmospheres, including oxidizing, reducing, and carbon dioxide environments—a crucial attribute for numerous practical applications.
The findings hold the potential to lay the groundwork for novel proton conductors destined for PCFCs/PCECs, boasting unparalleled performance capabilities.
The proposed strategies and the discovery of BSM20 could have a significant impact on energy and environmental science and technology.
Masatomo Yashima, Professor, Tokyo Institute of Technology
Journal Reference
Saito, K., & Yashima, M. (2023). High proton conductivity within the “Norby gap” by stabilizing a perovskite with disordered intrinsic oxygen vacancies. Nature Communications. doi.org/10.1038/s41467-023-43122-4.