Researchers at Oak Ridge National Laboratory, part of the Department of Energy, have made significant advances in their knowledge of an effective approach for direct air capture, or DAC, of carbon dioxide. With the goal of reaching negative emissions—where the amount of carbon dioxide extracted from the envelope of gases surrounding Earth surpasses the amount emitted—this DAC process is still in its early stages of development.
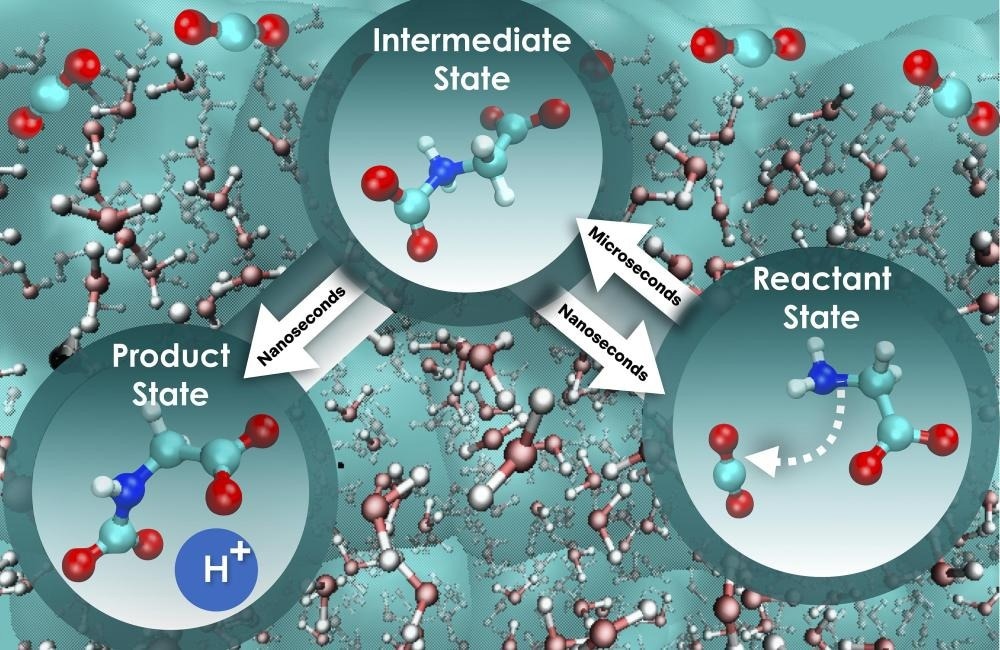 The research team combined a series of advanced computational methods to probe less-explored dynamic phenomena in liquid solutions related to the rate at which carbon dioxide can be captured. The carbon capture process is illustrated above. Image Credit: Santanu Roy/ORNL, US Dept. of Energy
The research team combined a series of advanced computational methods to probe less-explored dynamic phenomena in liquid solutions related to the rate at which carbon dioxide can be captured. The carbon capture process is illustrated above. Image Credit: Santanu Roy/ORNL, US Dept. of Energy
The fundamental processes of carbon dioxide sequestration utilizing aqueous glycine—an amino acid well-known for its absorbent properties—were the subject of the recently published study. The scientists investigated less-studied dynamic processes in liquid solutions connected to the rate at which carbon dioxide can get trapped by integrating several cutting-edge computational techniques.
Chemical reactions in water are complicated, especially when the motion of water molecules plays a big role. Water molecules and chemicals engage in something similar to a coupled dance that can marginally or significantly slow the reaction. Understanding these dynamic interactions, known as nonequilibrium solvent effects, is essential to getting the full picture of how reactions work and how fast they happen.
Santanu Roy, R&D Staff Member, Oak Ridge National Laboratory
The researchers determined that relying merely on the free energy barrier—the energy threshold that must be exceeded for a system to shift from one state to another—is an oversimplification that does not offer the entire picture when analyzing the pace at which carbon dioxide is absorbed. This haphazard approach could result in an incorrect understanding of reaction kinetics, or the factors that impact the pace at which a reaction happens.
We employed a more complete approach that considers the influence of water on the motion along the reaction path, and the outcome was intriguing. The initial step, where glycine interacts with carbon dioxide, is nearly 800 times slower compared with the next step, where a proton is released to ultimately form a mixture of product state for holding the absorbed carbon dioxide. Strikingly, the free energy barrier remains constant for both steps, and so this different perspective truly sets the speed of these two critical stages apart and offers a pathway to boost the efficiency of carbon dioxide absorption and separation.
Vyacheslav S. Bryantsev, R&D Staff, Oak Ridge National Laboratory
The comprehensive ab initio molecular dynamics simulations performed in this study were nonetheless hampered by the chemical processes’ short time and length scales and high computational costs.
For future projects, we intend to combine the emerging machine-learning approach with highly accurate simulations and develop interatomic interaction potentials based on deep neural networks. This will allow us to perform molecular simulations with high accuracy at large scales with significantly reduced computational costs.
Xinyou Ma, Postdoctoral Research Associate, Oak Ridge National Laboratory
Roy added, “While we have portrayed a molecular-level kinetics picture of carbon dioxide capture by aqueous amino acids, accessing large length and time scales through the use of the machine-learning approach will help us understand the effects of macroscopic factors such as temperature, pressure and viscosity on DAC and how these effects are related to the attained molecular picture.”
Overall, the findings offer insight into the complicated workings of DAC and highlight the critical role of kinetics, thermodynamics, and molecular interactions in aqueous amino acid carbon dioxide removal from the environment.
The potential of deploying a large-scale DAC system will become more practical as these mechanisms are well understood. Several DAC initiatives are in various phases of study, testing, and development across the world.
The Office of Science of the Department of Energy funded this study. The investigation made use of resources from ORNL’s Compute and Data Environment for Science, which is funded by the DOE Office of Science. This study also made use of the National Energy Research Scientific Computing Center, a DOE Office of Science user facility at Lawrence Berkeley National Laboratory.
Journal Reference:
Ma, X, et. al. (2023) An ab initio free energy study of the reaction mechanism and rate-limiting steps of CO2 capture by aqueous glycine. Cell Reports Physical Science. doi:10.1016/j.xcrp.2023.101642.