
This article discusses how Renishaw’s inVia Raman microscope is used to chemically characterize lithium-ion batteries. inVia is the ultimate analysis tool for studies ranging from fundamental materials research through to quality control and failure analysis of final products.
The inVia confocal Raman microscope
- Provides unambiguous identification of all types of target materials – inorganic, organic, amorphous, crystalline, gases, liquids and solids including polytype/allotrope differentiation
- Is capable of mapping rough and uneven surfaces
- Employs a non-destructive, non-contact technique
- Allows ex situ, in situ and operando measurements
- Is capable of resolving sub-micrometer features
- Delivers information on a material’s spatial distribution via 2D and 3D maps and depth profiles
- Automatically maintains focus during dynamic analysis of contracting and expanding surfaces as batteries are cycled
- Can be used along with atomic force microscopes for ultra-high resolution measurements
Identify Lithium-Ion Battery Components
- Polymers
- Metal oxides
- Electrolytes, ionic liquids
- Carbon materials
Correlation of Composition and Structure with Performance
- Detect structural changes
- Study the component distribution on the electrode surface or in cross-section
- Discriminate between spatially close components
It is possible to quantify results with metrics, such as particle statistics and fraction estimates.
In situ Electrochemical Characterization
inVia can be used with a wide selection of electrochemical cells and sample chambers. This aspect, in combination with an ability to be triggered from instruments such as potentiostats, allows for in situ and operando measurements.
There are also custom systems for inert gas glove boxes available.
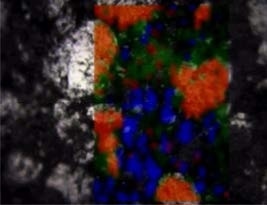
Raman map of an anode, superimposed on an optical microscope image. The map reveals graphite (red), acetyl black (blue) and hard carbon (green). Their relative abundances are, respectively: 13%, 10%, and 77%.
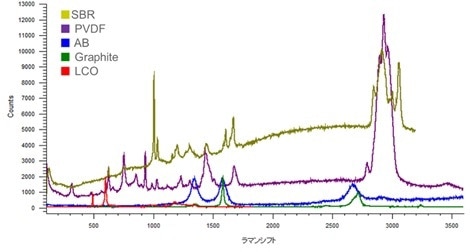
Detecting and identifying all the materials present, even at low concentration. Spectra taken in situ from: SBR (styrene-butadiene rubber, binder); PVDF (polyvinylidene fluoride, binder); AB (acetyl black, anode); graphite (anode); LCO (lithium cobalt oxide, cathode).
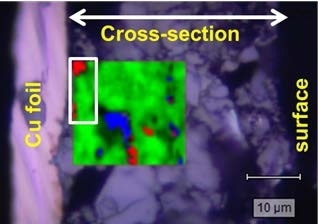
Detecting low concentrations of binder. Raman map of an anode (superimposed on an optical microscope image). The colors represent: SBR styrene-butadiene rubber binder (red); graphite (green); acetyl black (blue). The relative concentrations, as determined by the map, are, respectively: 1%, 97% and 2%.
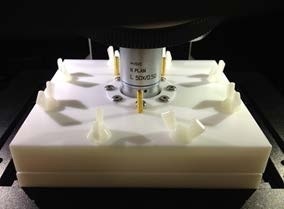
Using an electrochemical cell to perform operando measurements on a lithium-ion battery. Image courtesy of Mr. Hamura, Shimadzu Technoresearch, Japan.
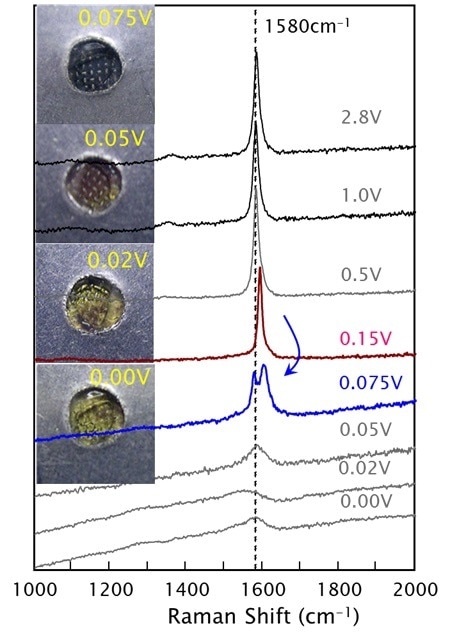
Operando studies of an anode. As the potential is changed, the anode’s appearance changes. The graphite G-band Raman peak also changes, indicating intercalation of lithium (shifting the peak to higher wavenumbers) and then a peak-splitting reflecting the intercalation penetrating to interior layers, rather than just the boundary layers. Data courtesy of Prof. Y. A. Kim, Shinshu University, Japan.
These results demonstrate the ease with which one can use Renishaw’s inVia confocal Raman microscope to analyze lithium-ion batteries.
inVia - The Ideal Lithium-Ion Battery Analysis Tool
- Research grade Raman microscope
- A range of rapid mapping and imaging solutions
- High confocality to scrutinize small details
- High sensitivity to detect traces of material
- Automation options, such as triggering of data acquisition by external systems (e.g. potentiostats)
- Raman-compatible electrochemical test cells available

This information has been sourced, reviewed and adapted from materials provided by Renishaw plc.
For more information on this source, please visit Renishaw plc.