Ammonia is among the top 10 chemicals produced today; fertilizers in one form or another account for 85% of the ammonia produced while other industrial uses include fibers, plastics, coatings and resins.

Image Credit: Shutterstock/saoirse2013
Most ammonia is produced by steam methane reforming of a natural gas feedstock. To improve process efficiency and reduce costs, manufacturers have adopted a closed loop control strategy: key to its success is the Process Insights MAX300-RTG real-time gas analyzer. The MAX300-RTG is a rugged quadrupole mass spectrometer employing sophisticated electronics and offering fully automatic operation. The universal analyzer offers stable real time data acquisition and a dynamic range of 10 ppb to 100%. The exact concentrations of the stream components are immediately available for integration into the control scheme.
Thanks to its reliability, speed and accuracy, the MAX300-RTG delivers unparalleled performance in ammonia production. It allows for tight control of four important process variables: the steam to carbon ratio, the H2:N2 ratio, methane slippage and process inert gases. Keeping tight control of these variables results in three significant economic benefits to ammonia producers: lower fuel costs, higher yields and an overall optimized efficiency. Figure 1 is a simple diagram of the ammonia process illustrating the typical streams analyzed by the MAX300-RTG.
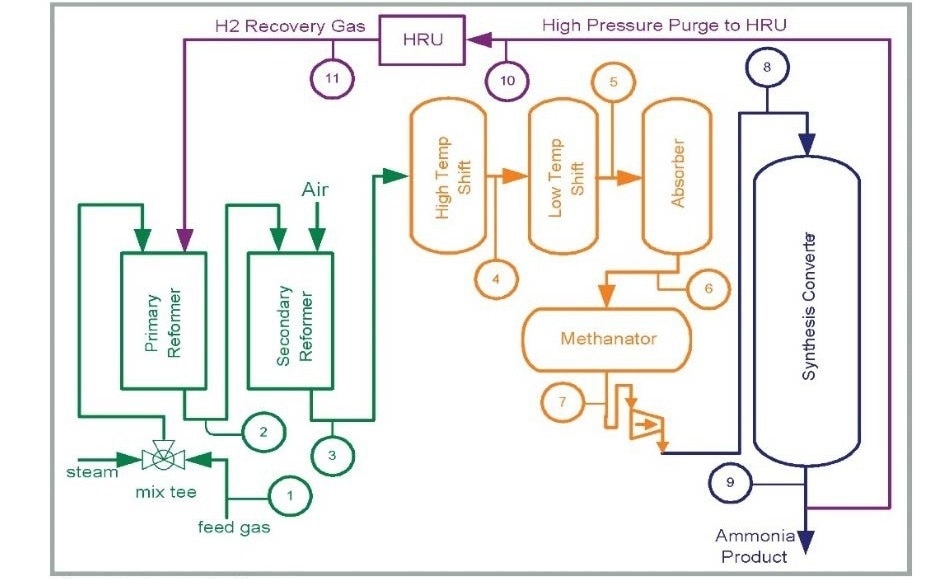
Figure 1. Ammonia Process
Feed Gas
In the Primary and Secondary Reformers, a hydrocarbon feed stream and steam are combined and passed over a reformer containing a nickel-based catalyst at 1500 °F. This leads to the formation of carbon monoxide and hydrogen: CH4 + H2O → CO + 3H2.
Known as coking, this carbon formation is detrimental to the catalyst. An excess amount of steam is mixed with the feed gas to prevent coking: a minimum ratio of 3:1 should be maintained i.e. 3.0 lb. moles of steam to 1.0 lb. moles of carbon. Plants usually operate with generously elevated steam to carbon ratios to prevent dropping below this ratio and degrading the catalyst.
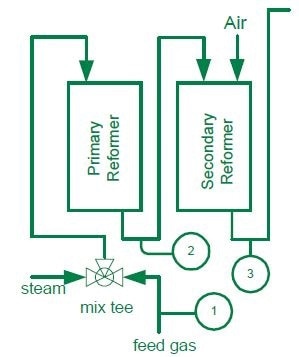
Control steam Generation Costs through Control of the Steam to Carbon Ratio to a tolerance of ± 0.02%.
One of the most expensive processes in ammonia production is generating steam: it is typically produced at a level four times that of the ammonia product. Tight control of the steam to carbon ratio can significantly decrease production costs.
The MAX300-RTG carries out rapid analysis of the feed stream for hydrocarbon concentration and calculates the BTU value. This information is used to regulate the steam flow; enabling rapid adjustments and avoiding potential converter problems. The MAX300-RTG typically analyzes hydrocarbons up to and including hexane in a Natural Gas Feed Stream, with the ability to analyze butane and pentane isomers separately. This results in greater accuracy for the calculated BTU value. Using the MAX300-RTG as part of a closed loop strategy, Steam to Carbon ratio tolerances of ± 0.02% have been demonstrated. Using the dual detector option, the MAX300-RTG can also analyze the Feed Gas stream for the presence of sulfur containing components such as hydrogen sulfide and mercaptans which are poisons and will de-activate the catalyst.
| Feed Gas |
| Component |
% Conc. |
| Nitrogen |
0.30 |
| Carbon Dioxide |
2.30 |
| Methane |
81.00 |
| Ethane |
9.60 |
| Propane |
4.10 |
| n-Butane |
1.10 |
| i-Butane |
0.40 |
| n-Pentane |
0.35 |
| i-Pentane |
0.15 |
| Hexane |
0.05 |
| Hydrogen Sulfide |
5 ppm |
Primary Reformer Effluent and Secondary Reformer Effluent
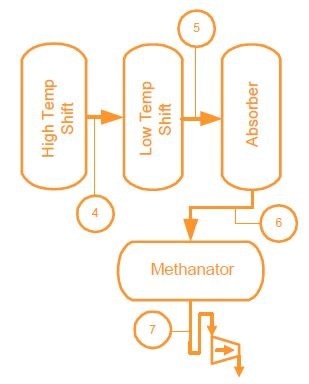
The quantity of un-reacted methane in the Primary Reformer Effluent and Secondary Reformer Effluent is referred to as methane slippage or leakage. It is an indication of the reformer efficiency in the conversion of methane to hydrogen and carbon dioxide. The control objective is to ensure the methane slippage from the secondary reformer at a very low and constant value, typically 0.3% methane or less.
Analysis of the Primary and Secondary Reformers necessitates both a highly sensitive and very stable sensor because of the complex chemical makeup. A wide dynamic range is also required because the methane conversion begins at 90% methane and ends at 0.3% methane. The sensitivity and the stability of the MAX300-RTG enables control tolerances of 0.05% to be achieved, allowing the operator to minimize the changes in methane slippage, thereby maximizing yield and lengthening equipment life. Increases of up to 20% in run time, and equipment life have been seen in plants employing closed loop control with an Process Insights CMS MAX300-RTG.
Maximize Yield and Equipment Life through Unsurpassed Control of Methane Slippage
Air provides the nitrogen required for ammonia reaction: it is introduced at the secondary reformer in an amount to yield an exit gas that has a 3:1 hydrogen to nitrogen ratio. The oxygen in the air reacts with the hydrocarbon feedstock in combustion and provides the energy to reform the remaining gas. The secondary reformer reaction increases the hydrogen content and oxidizes most of the CO to CO2, the latter being preferred as it is much more soluble in water and can easily be removed further along in the process. The Secondary Reformer Outlet also contains a small amount of methane and argon which enter the synthesis loop as inert gases.
CH4 + H2O → CO + 3H2 + (CH4)
H2 + Air → 3H2 + N2 + (CO2 + CO)
| Primary Reformer Effluent |
| Component |
% Conc. |
| Hydrogen |
67.00 |
| Nitrogen |
1.50 |
| Carbon Dioxide |
11.50 |
| Carbon Monoxide |
8.00 |
| Methane |
12.00 |
| Argon |
0.10 |
| Secondary Reformer Effluent |
| Component |
% Conc. |
| Hydrogen |
57.50 |
| Nitrogen |
22.50 |
| Carbon Dioxide |
8.50 |
| Carbon Monoxide |
12.00 |
| Methane |
0.30 |
| Argon |
0.30 |
High Temperature Shift, Low Temperature Shift (CO Removal)
To maximize hydrogen yield, more steam is added to convert carbon monoxide to carbon dioxide and hydrogen. The reaction takes place in shift converters at high temperatures over an iron-oxide based catalyst. The mass spectrometer usually measures both the High Temperature Shift and the Low Temperature Effluents.
CO + H2O → CO2 + H2
| High Temperature Shift |
| Component |
% Conc. |
| Hydrogen |
52.70 |
| Nitrogen |
27.27 |
| Carbon Dioxide |
14.53 |
| Carbon Monoxide |
3.60 |
| Methane |
1.55 |
| Low Temperature Shift |
| Component |
% Conc. |
| Hydrogen |
54.20 |
| Nitrogen |
26.40 |
| Carbon Dioxide |
17.19 |
| Carbon Monoxide |
0.40 |
| Methane |
1.50 |
| Argon |
0.35 |
CO2 Absorber and Methanator
CO2 is removed by absorber-regenerator units containing many types of absorbent including monoethanolamine solution (MEA), sulfinol, propylene carbonate amongst others. After this process, CO in the product gas is less than 100 ppm.
Collectively known as carbon oxides, CO2 and CO are poisonous to many types of catalysts and trace amounts of both present after the absorber must be removed from the synthesis gas. This is achieved by converting them to methane over a nickel or ruthenium catalyst in the presence of hydrogen in the Methanator. The amount of methane produced by this process is insignificant compared to the level of the un-reacted methane slip. Residual levels of the carbon oxides in the process gas will be less than 5 ppm.
CO + 3H2 → CH4 + H2O
CO2 + 4H2 → CH4 + 2H2O
| Absorber Outlet |
| Component |
% Conc. |
| Hydrogen |
65.33 |
| Nitrogen |
31.80 |
| Carbon Dioxide |
0.08 |
| Carbon Monoxide |
0.48 |
| Methane |
1.81 |
| Argon |
0.41 |
| Argon |
0.35 |
| Methanator Outlet |
| Component |
% Conc. |
| Hydrogen |
69.80 |
| Nitrogen |
28.00 |
| Carbon Dioxide |
< 5 ppm |
| Carbon Monoxide |
< 5 ppm |
| Methane |
1.70 |
| Argon |
0.30 |
Converter Inlet, Converter Outlet
Critical to the efficient production of ammonia is controlling the H2:N2 ratio at the Synthesis Converter Inlet. With the speed and stability of the MAX300RTG and a closed loop strategy, the H2:N2 ratio at the Converter Inlet can be maintained within a tolerance of ± 0.01%. Using a gas chromatograph, the tolerance is typically 0.10%.
Control the Feed to Air H2:N2 Ratio within ± 0.01%
Estimates suggest that utilizing the MAX300-RTG on the four critical streams for the H2:N2 ratio control and mass balance can produce an additional 10.3 tons of ammonia per day in a 1000 ton-per-day plant. This additional output is in comparison to the expected plant output when using a gas chromatograph with its typical 5-6-minute cycle time per stream.
N2 + 3H2 → 2NH3
| Converter Inlet |
| Component |
% Conc. |
| Hydrogen |
65.00 |
| Nitrogen |
22.50 |
| Argon |
2.50 |
| Helium |
0.50 |
| Methane |
7.00 |
| Ammonia |
2.00 |
| Converter Outlet |
| Component |
% Conc. |
| Hydrogen |
54.00 |
| Nitrogen |
19.50 |
| Argon |
3.50 |
| Helium |
0.50 |
| Methane |
7.50 |
| Ammonia |
15.00 |
High Pressure Purge and HRU
The control of process inert gases - argon, methane and helium - is another important benefit offered by the MAX300-RTG mass spectrometer. The control of these gases is essential in maintaining constant converter feed ratios. Much of the converter feed make up is a result of recycled converter gases after ammonia production. Analyzes of the Converter Inlet, Converter Recycle and High Pressure Purge Loop with the mass spectrometer ensures these inerts can be maintained within a tolerance of ± 0.01%.

Maintain Constant Converter Feed Ratios through the Control of Process Inert Gases to ± 0.01%
| High Pressure Purge to HRU |
| Component |
% Conc. |
| Hydrogen |
62.00 |
| Nitrogen |
22.50 |
| Argon |
3.50 |
| Helium |
0.50 |
| Methane |
11.00 |
| Ammonia |
2.00 |
| H2 Recovery Gas |
| Component |
% Conc. |
| Hydrogen |
50.00 |
| Nitrogen |
10.00 |
| Argon |
1.75 |
| Helium |
0.60 |
| Methane |
37.50 |
Conclusion
In the past few years, efficient process control has become even more important in ammonia production as the cost of raw materials have increased and the product price has decreased: to improve process efficiency and reduce costs, manufacturers have adopted closed loop control strategies. Ammonia producers must take advantage of every available opportunity to improve their process. Key to an efficacious closed loop control strategy is the analyzer used to supply the Distributed Control System (DCS) with information: they must be fast, reliable, accurate, precise and rugged. Among ammonia producers, the Process Insights CMS Process Mass Spectrometer has developed a reputation as the “must have” analyzer for the closed loop control of the ammonia process.
The Process Insights CMS MAX300-RTG Process Mass Spectrometer has a proven record of performance and reliability. It’s real time data acquisition, allows for 12 sample points to be reported in less than two minutes. In particular, the mass spectrometer provides information which allows tight control of four critical process variables:
- The steam to carbon ratio can be controlled to a tolerance of ± 0.02% by an accurate and fast analysis of the feed gas stream, significantly reducing steam generation costs.
- Controlling the methane slippage at the reformer outlet results in a maximum product yield and extends equipment life.
- Production efficiency is increased by controlling the feed to air (H2:N2) ratio at the Synthesis Converter Inlet to within ± 0.01%.
- Efficiency can be improved by maintaining constant converter feed ratios through control of the process inert gases within 0.01%.


This information has been sourced, reviewed and adapted from materials provided by Process Insights - Mass Spectrometry.
For more information on this source, please visit Process Insights – Mass Spectrometers.