The EDGE® automated solvent extraction system is the most state-of-the-art and innovative extraction system available. By uniting pressurized fluid extraction and dispersive solid phase extraction, the EDGE has the capacity to significantly reduce the sample preparation time and limit the potential for human error.

Image Credit: CEM Corporation
The result is a fast, efficient and simple extraction. In around 15 minutes, phthalates can be extracted from various types of plastics, including polyvinyl chloride (PVC). This incorporates sample cooling and filtration, as well as system washing.
Introduction
Phthalates are hazardous chemical toxins that can induce damage to the liver, kidneys, lungs and reproductive system and have been associated with alterations in DNA integrity. They have been used since the 1950s to soften plastics and are found in everyday products, including vinyl flooring, personal care products, backpacks, children’s lunch boxes and toys.
The major concern is the presence of phthalates in products marketed towards and used by young children, who are more likely to put items in their mouths. Due to the fact they are not chemically bound to the plastic, phthalates continually leach into the environment, and people are exposed through frequent contact.
Therefore, extracting phthalates from plastics must be a quick and simple process so that manufacturers can release products with the confidence that they meet the safety standards of the consumer product safety commission (CPSC). Extracting phthalates from plastics is a complex process for many reasons.
Firstly, the low melting point of plastic polymers means extraction is difficult. Plastics must be reformed without melting to produce a good extraction, and achieving this balance can be a challenge.
Also, due to the complexity of samples, existing extraction methods, such as Soxhlet and pressurized fluid extraction (both referenced in CPSC-CH-C1001-09.3), often generate extracts that are muddy and contain a number of co-extracts, making analysis difficult.
Additionally, the conventional extraction methods, as referenced in CPSC-CH-C1001-09.3, can be time-sapping and laborious, which necessitate large volumes of solvent.
The EDGE has the capacity to produce a clean, filtered and cooled extract that is prepared for analysis in under 15 minutes, requiring no more than 50 mL of solvent. Each 15-minute extraction cycle also includes an effective solvent wash protocol that cleans the system and reduces the risk of carryover.
Materials and Methods
Reagents
A polyvinyl chloride CRM-PVC001 was acquired from SPEX CertiPrep. The CRM contained the following phthalates of interest: bis(2-ethylhexhyl) phthalate, butylbenzyl phthalate, diethyl phthalate, dimethyl phthalate, di-n-butyl phthalate and di-noctyl phthalate.
For the extraction, rinse and wash, a solvent made up of a 50/50 mixture of isopropanol/cyclohexane was used. Three standards acquired from SPEX CertiPrep were utilized to create a calibration curve for each phthalate of interest, dimethyl phthalate in methanol (S-1590), diethyl phthalate in methanol (S-1515) and phthalate standard in isooctane (C1001-09).
Sample Preparation
An EDGE system was used for extracting, filtering and cleaning all samples. Subsequently, a S1 Q-Disc® stack (C9+G1+C9 sandwich) was placed into the Q-Cup® base for each sample. The two parts were then fastened together, producing a seal between the Q-Cup and Q-Disc. One gram of polyvinyl chloride was weighed and poured into a pre-assembled Q-Cup.
A Q-Screen® was positioned on top of each sample via a Q-Screen tool. The Q-Cups were fixed into the removable rack of the EDGE – each of which has a collection vial – and the rack was slipped in place on the EDGE and then queued for extraction utilizing the CEM-approved method parameters as described below.
EDGE Method for the Extraction of Pthalates from PVC
Q-Disc: S1 Q-Disc stack (C9+G1+C9 sandwich)
Cycle 1:
- Extraction Solvent: IPA/cyclohexane (1:1)
- Top Add: 20 mL
- Bottom Add: 10 mL
- Rinse: 0 mL
- Temperature: 80 ºC
- Hold Time: 10:00 (mm:ss)
Wash 1:
- Wash Solvent: IPA/cyclohexane (1:1)
- Wash Volume: 10 mL
- Temperature: 80 ºC
- Hold Time: 00:15 (mm:ss)
Wash 2:
- Wash Solvent: IPA/cyclohexane (1:1)
- Wash Volume: 10 mL
- Temperature: - - -
- Hold Time: - -:- -
Analysis
IPA/cyclohexane was used to dilute extract samples to 50 mL with an equal ratio (1:1). An aliquot of each extract was inserted into an Agilent 7890A with a 5975C MSD for analysis in compliance with EPA 8270. A Phenomenex ZB-5MSplus 30 m, 0.25 mm column was utilized.
Results
Figure 1 is indicative of a GCMS chromatogram displaying clean separation of the phthalates of interest; the retention times of the analytes of interest are shown in Table 1. For all phthalates of interest, recovery data was detected via 8-point calibration curves at 12, 24, 48, 60, 80 and 100 ppm.
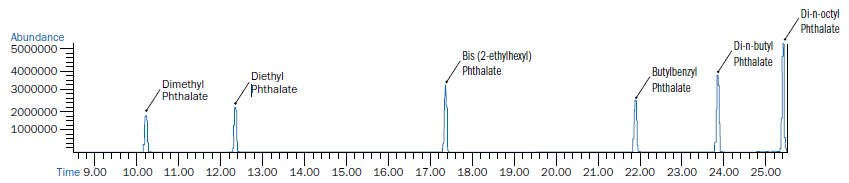
Figure 1. GCMS Chromatogram of Phthalates of Interest. Image Credit: CEM Corporation
Table 1. Retention Times of Phthalates of Interest. Source: CEM Corporation
| Standard Compound |
Retention Time (min) |
| Dimethyl Phthalate |
10.297 |
| Diethyl Phthalate |
12.375 |
| Bis (2-ethylhexyl) Phthalate |
17.305 |
| Butylbenzyl Phthalate |
21.758 |
| Di-n-butyl Phthalate |
23.223 |
| Di-n-octyl Phthalate |
25.223 |
All calibration curves possess R2 values exceeding 0.99. For each of the phthalates of interest, the eventual concentration of the extracts was 60 ppm. Table 2 highlights the percent recovery of the extraction of phthalates from polyvinyl chloride.
Table 2. Percent Recovery Data for Polyvinyl Chloride. Source: CEM Corporation
| Standard Compound |
Average Recovery (n=6) |
RSD |
| Dimethyl Phthalate |
86 |
1.9 |
| Diethyl Phthalate |
83 |
1.5 |
| Bis (2-ethylhexyl) Phthalate |
88 |
1.5 |
| Butylbenzyl Phthalate |
86 |
1.7 |
| Di-n-butyl Phthalate |
84 |
2.5 |
| Di-n-octyl Phthalate |
101 |
1.7 |
All methods for sample preparation, extraction and analysis were predicated on CPSC-CH-C1001-09.1. The entire method, including the extraction process, extraction, filtration and system washing, never surpassed 15 min. The final extraction volume was 30 mL, and an additional 20 mL of solvent was for washing the system.
Conclusion
To achieve an acceptable recovery range, the percent recovery data should be within 80-120% for the extraction of phthalates from plastics.
The EDGE produced appropriate recoveries for the extraction of phthalates from polyvinyl chloride. Additionally, the EDGE tackled all the constraints of existing extraction methods. As a result of precise temperature control, the low melting point of polyvinyl chloride (PVC) was not a worry.
The Q-Disc facilitated fine filtration of the sample, which minimized interference from other contaminates, generating clean and easy-to-analyze extracts. The EDGE method consumed less solvent and time than the extraction methods mentioned in CPSC-CH-C1001-09.3.
Finally, since the EDGE is an automated system, it circumvents the human error affiliated with other extraction techniques. The EDGE has the capacity to extract phthalates accurately and economically from plastics.

This information has been sourced, reviewed and adapted from materials provided by CEM Corporation.
For more information on this source, please visit CEM Corporation.