Sponsored by HORIBAJun 12 2018
Pulsed RF Glow Discharge Optical Emission Spectrometry facilitates ultra-fast elemental depth profiling. It could also be used in the characterization of both positive and negative Li-ion battery electrodes.
Introduction
A lithium-ion (Li-ion) battery is a rechargeable battery wherein lithium ions move freely between the anode and the cathode. This movement generates electricity. Chemical reactions involving the interaction of particles do not occur at the surface of the electrodes; instead, they affect depths as far as tens of micrometers. Pulsed RF Glow Discharge Optical Emission Spectrometry provides ultra-fast elemental depth profiling of thin and thick films. The device has been empirically proven to be a successful tool in the characterization of positive and negative electrodes of lithium-ion batteries.
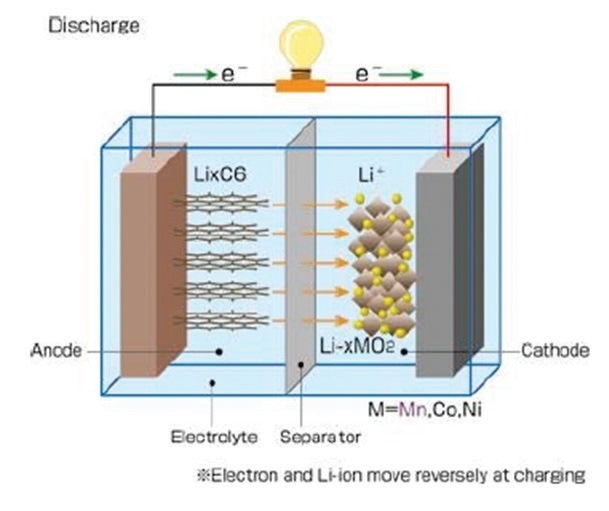
Image Credit: Automotive Energy Supply Corporation, 2007
HORIBA has more than 15 global references in such field. Experimental results are generally protected by non-disclosure agreements, and as such, could not be communicated.
Instrumentation
The GD Profiler 2 utilizes an advanced Pulsed RF Glow Discharge Source to a high resolution, wide spectral range Optical Emission Spectrometer.
The GD plasma source allows for the precise and fast investigation of a representative part of the electrode. This part would typically be 4mm in diameter. Pulsed RF operation is a relevant tool in avoiding damage of fragile electrodes and in preventing unwanted diffusion during measurements. At the same time, sputtered particles are excited by the same GD plasma. As a focal function of the sputtered depth, the spectrometer simultaneously provides a measure of all relevant elements.

GD Profiler 2
Typical Results
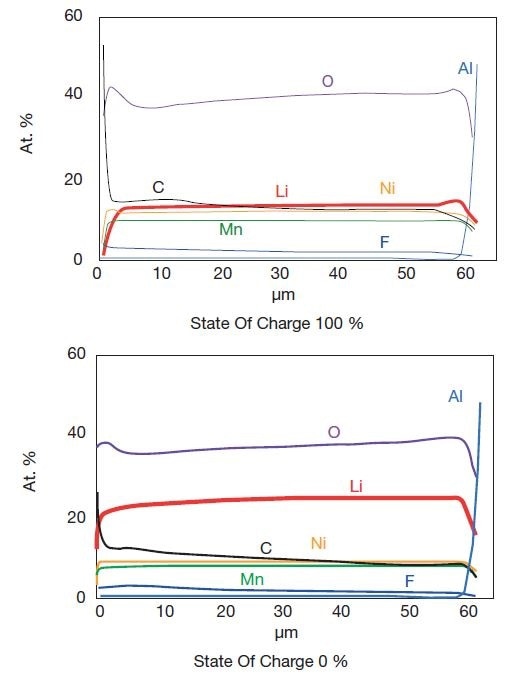
The following graph presents a detailed depth profile analysis of the positive electrode when a battery is fully charged and discharged.
Key Features
The use of Pulsed RF Glow Discharge Optical Emission Spectrometry yields ultra-fast depth profiling of positive and negative electrodes, yielding a typical erosion rate of several microns per minute. The device also features fast sputtering that yields high sample throughput and higher sensitivity. As a result, more sputtered materials are excited over a given period of time. Apart from these key features, the following qualities also characterize the Pulsed RF Glow Discharge Optical Emission Spectrometry:
- Easy to use as the GD source does not require UHV
- Simultaneous measurement of all elements involved in analysis
- Mean information provided over the sputtered area
- Lack of thermal effects when measuring using Pulsed RF operation
- Provide quick access to embedded interfaced to facilitate further SEM observation
Sample Handling Strategies
Oftentimes, electrodes are soft and fragile. As such, analysis of these particles should be dealt with utmost care and specificity. HORIBA has developed successful and appropriate strategies to efficiently analyze such electrodes, including a patented transfer chamber called “Li bell” for flammable ones.
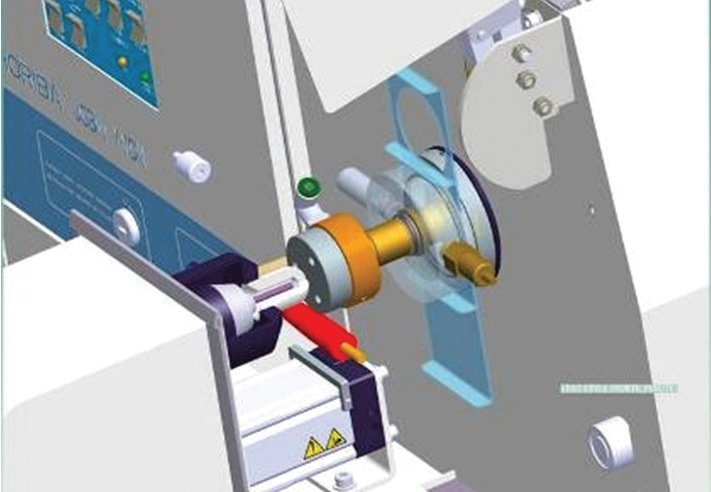
Schematic view of the Li bell mounted on the GD Profiler 2 instrument
Conclusion
The Pulsed RF Glow Discharge Optical Emission Spectrometry is designed to efficiently facilitate multiple processes involved in studying electrodes—including, but not limited to: coating behaviors, charge and discharge processes, process control, or comparative studies.
Sources and Further Reading
- Yoshiyasu Saito, Md. Khalilur Rahman. (2007). Investigation of positive electrodes after cycle testing of high-power Li-ion battery cells IV. An approach to the power fading mechanism by depth profile analysis of electrodes using glow discharge optical emission spectroscopy. Journal of Power Sources, 174, 877–882.
- Han Gab Song, Kyu-Sung Park, Yong Joon Park. (2012), The effects of LaPO4 coating on the electrochemical properties of Li[Ni0.5Co0.2Mn0.3]O2 cathode material. Solid State Ion. doi:10.1016/j.ssi.2011.12.014.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.