Various physical methods are commonly used for a chemical analysis of the solid surface. However, these methods often require a time-consuming sample preparation, or they are not sensitive enough for the outermost surfaces. The zeta potential is a characteristic parameter for describing the surface chemistry of solids. It is located at the interface between a solid and a surrounding liquid.
The zeta potential represents the surface charge, which occurs in the presence of an aqueous solution when reactive (functional) groups dissociate on hydrophilic surfaces or water ions adsorb onto hydrophobic surfaces. Varying the pH value of the aqueous phase influences the equilibrium between dissociation and adsorption processes, giving insights into the chemical behavior of the surface.
Surface Zeta Potential
The streaming potential and streaming current are measured to determine the zeta potential of macroscopic solid surfaces. An aqueous solution is set to flow across the solid surface under defined pressure conditions. The SurPASS™ 3 instrument by Anton Paar provides a comprehensive solution: With its range of different measuring cells SurPASS™ 3 determines the zeta potential of solids of various shape and size.
In the Cylindrical Cell, fibrous samples, powder or granules are arranged in a permeable layer. The measuring liquid streams through this fiber plug or powder bed (Figure 1). The differential pressure be-tween both sides of the fiber or powder sample is determined by the sample packing density. This can be adjusted reproducibly using the monitored flow behavior.
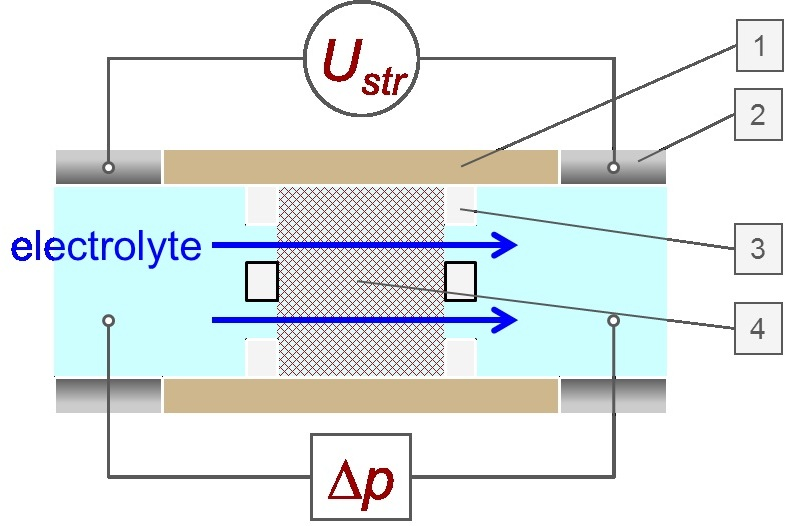
Figure 1. Schematic drawing of the Cylindrical Cell for fiber and powder samples (1 Cylindrical Cell, 2 electrode, 3 support disk, 4 sample plug).
In the measuring cells for samples with planar surfaces, i.e., the Clamping Cell and the Adjustable Gap Cell, a defined gap is set between two opposing sample surfaces. During the measurement the liquid flows through this gap and produces a pressure gradient and a charge separation at the solid/liquid interface. The streaming potential or streaming current is the electrical response to the shift in the surface charge.
In the Clamping Cell the height of the gap is defined by a spacer, whereas in the Adjustable Gap Cell the distance between samples is continuously adjusted (Figure 2). This allows investigations into the surface properties of samples with a rough surface, severe swelling behavior or a high degree of porosity.
Independent of the measuring cell used, the pressure difference is continuously decreased and the resulting streaming potential (or streaming current) is measured. The relationship between these two measuring parameters is linear (Figure 3), with the slope dUstr/dΔp or dlstr/dΔp being proportional to the zeta potential.
As a property of the interface between a solid and the surrounding liquid, the zeta potential is also influenced by the conductivity or electrolyte concentration of the liquid phase. A common electrolyte is an aqueous 0.001 mol/l solution of a simple salt such as KCl or NaCl. This allows a reproducible setting of the conductivity. The low electrolyte concentration also ensures a high sensitivity of the measuring method.
The preferred method for characterizing the solid surface is to vary the pH value of the aqueous solution and thus to carry out a titration of the surface. The dissociation of functional surface groups results in the formation of charge carriers on the surface. The number of these charge carriers changes with the pH value. This relationship allows qualitative insights into the chemistry of these functional groups. It is also possible to calculate the pK value of an acidic or basic surface entity. The integrated titration unit of Sur-PASS™ 3 automatically sets the pH value, e.g., to determine the isoelectric point.
The addition of chemicals to the aqueous solution (salts of multivalent ions, anionic or cationic surfactants, polyelectrolytes, proteins) gives further application-specific insights into the selective interactions of these components with the solid surface. For example, this allows investigation into the adsorption processes of surfactants on textile fibers or plastic surfaces. Another example is the change in the zeta potential of a filter membrane due to the selective adsorption of a divalent cation on its surface.

 Want to know more? Click here to read the full article.
Want to know more? Click here to read the full article.
.jpg)
This information has been sourced, reviewed and adapted from materials provided by Anton Paar GmbH.
For more information on this source, please visit Anton Paar GmbH.